Therapeutics and Immunoprophylaxis Against Noroviruses and Rotaviruses: The Past, Present, and Future
- PMID: 28901254
- PMCID: PMC5971199
- DOI: 10.2174/1389200218666170912161449
Therapeutics and Immunoprophylaxis Against Noroviruses and Rotaviruses: The Past, Present, and Future
Abstract
Background: Noroviruses and rotaviruses are important viral etiologies of severe gastroenteritis. Noroviruses are the primary cause of nonbacterial diarrheal outbreaks in humans, whilst rotaviruses are a major cause of childhood diarrhea. Although both enteric pathogens substantially impact human health and economies, there are no approved drugs against noroviruses and rotaviruses so far. On the other hand, whilst the currently licensed rotavirus vaccines have been successfully implemented in over 100 countries, the most advanced norovirus vaccine has recently completed phase-I and II trials.
Methods: We performed a structured search of bibliographic databases for peer-reviewed research literature on advances in the fields of norovirus and rotavirus therapeutics and immunoprophylaxis.
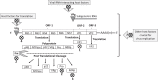
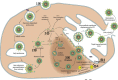
Results: Technological advances coupled with a proper understanding of viral morphology and replication over the past decade has facilitated pioneering research on therapeutics and immunoprophylaxis against noroviruses and rotaviruses, with promising outcomes in human clinical trials of some of the drugs and vaccines. This review focuses on the various developments in the fields of norovirus and rotavirus therapeutics and immunoprophylaxis, such as potential antiviral drug molecules, passive immunotherapies (oral human immunoglobulins, egg yolk and bovine colostral antibodies, llama-derived nanobodies, and antibodies expressed in probiotics, plants, rice grains and insect larvae), immune system modulators, probiotics, phytochemicals and other biological substances such as bovine milk proteins, therapeutic nanoparticles, hydrogels and viscogens, conventional viral vaccines (live and inactivated whole virus vaccines), and genetically engineered viral vaccines (reassortant viral particles, virus-like particles (VLPs) and other subunit recombinant vaccines including multi-valent viral vaccines, edible plant vaccines, and encapsulated viral particles).
Conclusions: This review provides important insights into the various approaches to therapeutics and immunoprophylaxis against noroviruses and rotaviruses.
Keywords: Gastroenteritis; antiviral molecules; norovirus; other therapeutic approaches; passive immunotherapy; rotavirus; vaccines..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis.Vaccine. 2011 Oct 19;29(45):8126-33. doi: 10.1016/j.vaccine.2011.08.026. Epub 2011 Aug 18. Vaccine. 2011. PMID: 21854823
-
Combination of three virus-derived nanoparticles as a vaccine against enteric pathogens; enterovirus, norovirus and rotavirus.Vaccine. 2019 Dec 3;37(51):7509-7518. doi: 10.1016/j.vaccine.2019.09.072. Epub 2019 Oct 1. Vaccine. 2019. PMID: 31585726
-
Generation of Recombinant Rotaviruses Expressing Human Norovirus Capsid Proteins.J Virol. 2022 Nov 23;96(22):e0126222. doi: 10.1128/jvi.01262-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314817 Free PMC article.
-
Viral gastroenteritis.Lancet. 2018 Jul 14;392(10142):175-186. doi: 10.1016/S0140-6736(18)31128-0. Epub 2018 Jun 29. Lancet. 2018. PMID: 30025810 Free PMC article. Review.
-
Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus.Nanomedicine (Lond). 2012 Jun;7(6):889-97. doi: 10.2217/nnm.12.62. Nanomedicine (Lond). 2012. PMID: 22734641 Free PMC article. Review.
Cited by
-
Reconstitution of Norovirus-Specific T-Cell Responses Following Hematopoietic Stem Cell Transplantation in Patients With Inborn Errors of Immunity and Chronic Norovirus Infection.J Infect Dis. 2025 Mar 17;231(3):773-783. doi: 10.1093/infdis/jiae398. J Infect Dis. 2025. PMID: 39140311 Free PMC article.
-
Norovirus in health care and implications for the immunocompromised host.Curr Opin Infect Dis. 2019 Aug;32(4):348-355. doi: 10.1097/QCO.0000000000000557. Curr Opin Infect Dis. 2019. PMID: 31107251 Free PMC article.
-
In Depth Breadth Analyses of Human Blockade Responses to Norovirus and Response to Vaccination.Viruses. 2019 Apr 26;11(5):392. doi: 10.3390/v11050392. Viruses. 2019. PMID: 31035476 Free PMC article.
-
Codon optimization, expression in Escherichia coli, and immunogenicity analysis of deformed wing virus (DWV) structural protein.PeerJ. 2020 Mar 11;8:e8750. doi: 10.7717/peerj.8750. eCollection 2020. PeerJ. 2020. PMID: 32201647 Free PMC article.
-
The Efficacy of Probiotics as Antiviral Agents for the Treatment of Rotavirus Gastrointestinal Infections in Children: An Updated Overview of Literature.Microorganisms. 2022 Dec 2;10(12):2392. doi: 10.3390/microorganisms10122392. Microorganisms. 2022. PMID: 36557645 Free PMC article. Review.
References
-
- de Graaf M., Van Beek J., Koopmans M.P. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016;14(7):421–433. - PubMed
-
- Ayukekbong J.A., Mesumbe H.N., Oyero O.G., Lindh M., Bergström T. Role of noroviruses as aetiological agents of diarrhoea in developing countries. J. Gen. Virol. 2015;96(8):1983–1999. - PubMed
-
- Patel M.M., Hall A.J., Vinjé J., Parashar U.D. Noroviruses: A comprehensive review. J. Clin. Virol. 2009;44(1):1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

