Immunocontraception: Filamentous Bacteriophage as a Platform for Vaccine Development
- PMID: 28901276
- PMCID: PMC5738698
- DOI: 10.2174/0929867324666170911160426
Immunocontraception: Filamentous Bacteriophage as a Platform for Vaccine Development
Abstract
Background: Population control of domestic, wild, invasive, and captive animal species is a global issue of importance to public health, animal welfare and the economy. There is pressing need for effective, safe, and inexpensive contraceptive technologies to address this problem. Contraceptive vaccines, designed to stimulate the immune system in order to block critical reproductive events and suppress fertility, may provide a solution. Filamentous bacteriophages can be used as platforms for development of such vaccines.
Objective: In this review authors highlight structural and immunogenic properties of filamentous phages, and discuss applications of phage-peptide vaccines for advancement of immunocontraception technology in animals.
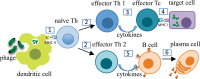
Results: Phages can be engineered to display fusion (non-phage) peptides as coat proteins. Such modifications can be accomplished via genetic manipulation of phage DNA, or by chemical conjugation of synthetic peptides to phage surface proteins. Phage fusions with antigenic determinants induce humoral as well as cell-mediated immune responses in animals, making them attractive as vaccines. Additional advantages of the phage platform include environmental stability, low cost, and safety for immunized animals and those administering the vaccines.
Conclusion: Filamentous phages are viable platforms for vaccine development that can be engineered with molecular and organismal specificity. Phage-based vaccines can be produced in abundance at low cost, are environmentally stable, and are immunogenic when administered via multiple routes. These features are essential for a contraceptive vaccine to be operationally practical in animal applications. Adaptability of the phage platform also makes it attractive for design of human immunocontraceptive agents.
Keywords: Animal population control; bacteriophage; contraception; phage immunogenicity; phage-peptide fusions; vaccine..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures

Similar articles
-
Infective and inactivated filamentous phage as carriers for immunogenic peptides.J Virol Methods. 2012 Jul;183(1):63-8. doi: 10.1016/j.jviromet.2012.03.032. Epub 2012 Apr 3. J Virol Methods. 2012. PMID: 22575687
-
Generation and characterization of phage-GnRH chemical conjugates for potential use in cat and dog immunocontraception.Reprod Domest Anim. 2012 Dec;47 Suppl 6:406-11. doi: 10.1111/rda.12061. Reprod Domest Anim. 2012. PMID: 23279551
-
Architectural insight into inovirus-associated vectors (IAVs) and development of IAV-based vaccines inducing humoral and cellular responses: implications in HIV-1 vaccines.Viruses. 2014 Dec 17;6(12):5047-76. doi: 10.3390/v6125047. Viruses. 2014. PMID: 25525909 Free PMC article. Review.
-
Construction of a filamentous phage display peptide library.Methods Mol Biol. 2014;1088:19-33. doi: 10.1007/978-1-62703-673-3_2. Methods Mol Biol. 2014. PMID: 24146394
-
The development of inovirus-associated vector vaccines using phage-display technologies.Expert Rev Vaccines. 2019 Sep;18(9):913-920. doi: 10.1080/14760584.2019.1651649. Epub 2019 Sep 8. Expert Rev Vaccines. 2019. PMID: 31373843 Free PMC article. Review.
Cited by
-
Detection of Acidic Pharmaceutical Compounds Using Virus-Based Molecularly Imprinted Polymers.Polymers (Basel). 2018 Sep 1;10(9):974. doi: 10.3390/polym10090974. Polymers (Basel). 2018. PMID: 30960899 Free PMC article.
-
Comprehensive Evaluation and Future Perspectives of Non-Surgical Contraceptive Methods in Female Cats and Dogs.Animals (Basel). 2025 May 21;15(10):1501. doi: 10.3390/ani15101501. Animals (Basel). 2025. PMID: 40427377 Free PMC article. Review.
-
Phage constructs targeting gonadotropin-releasing hormone for fertility control: evaluation in cats.J Feline Med Surg. 2020 Aug;22(8):685-695. doi: 10.1177/1098612X19875831. Epub 2019 Sep 30. J Feline Med Surg. 2020. PMID: 31566070 Free PMC article.
-
Mass spectrometry enumeration of filamentous M13 bacteriophage.Anal Biochem. 2019 Oct 1;582:113354. doi: 10.1016/j.ab.2019.113354. Epub 2019 Jul 2. Anal Biochem. 2019. PMID: 31276652 Free PMC article.
-
Bacteriophage T4 nanoparticles for vaccine delivery against infectious diseases.Adv Drug Deliv Rev. 2019 May;145:57-72. doi: 10.1016/j.addr.2018.06.025. Epub 2018 Jul 6. Adv Drug Deliv Rev. 2019. PMID: 29981801 Free PMC article. Review.
References
-
- Gupta S.K., Srinivasan V.A., Suman P., Rajan S., Nagendrakumar S.B., Gupta N., Shrestha A., Joshi P., Panda A.K. Contraceptive vaccines based on the zona pellucida glycoproteins for dogs and other wildlife population management. Am. J. Reprod. Immunol. 2011;66(1):51–62. - PubMed
-
- Kirkpatrick J.F., Lyda R.O., Frank K.M. Contraceptive vaccines for wildlife: a review. Am. J. Reprod. Immunol. 2011;66(1):40–50. - PubMed
-
- Swegen A., Aitken R.J. Prospects for immunocontraception in feral horse population control: exploring novel targets for an equine fertility vaccine. Reprod. Fertil. Dev. 2016;28:853–863. - PubMed
-
- Ackermann H-W. Bacteriophages classification. In: Kutter E., Sulakvelidze A., editors. Bacteriophages: Biology and application. CRC Press; 2005. pp. 67–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

