miR‑21 attenuates contrast‑induced renal cell apoptosis by targeting PDCD4
- PMID: 28901491
- PMCID: PMC5865832
- DOI: 10.3892/mmr.2017.7426
miR‑21 attenuates contrast‑induced renal cell apoptosis by targeting PDCD4
Abstract
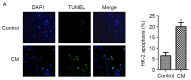
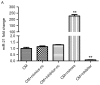
Contrast medium (CM) is widely used in cardiac catheterization; however, it may induce acute kidney injury or renal failure, although the underlying mechanism remains to be elucidated. MicroRNA‑21 (miR‑21) is involved in renal disease and has been indicated to regulate cellular apoptosis and fibrosis, although its role in CM‑induced renal cell injury is unknown. The present study examined the expression and potential targets of miR‑21 in human renal proximal tubular epithelial (HK‑2) cells following CM treatment. CM induced renal cell apoptosis and decreased miR‑21 expression. The expression level of the apoptosis regulator protein, B‑cell lymphoma 2 (Bcl‑2) was upregulated, whereas that of the apoptosis regulator, Bcl‑2‑associated X protein (Bax) was downregulated upon transfection of miR‑21 mimics; miR‑21 overexpression additionally directly inhibited the expression of programmed cell death protein 4 (PDCD4), as determined by a dual luciferase reporter assay, and PDCD4 silencing reduced the rate of HK‑2 cell apoptosis. The results of the present study indicated that miR‑21 protected renal cells against CM‑induced apoptosis by regulating PDCD4 expression.
Figures



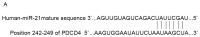
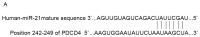
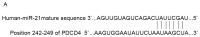


Similar articles
-
Role of miR-21 in the growth and metastasis of human salivary adenoid cystic carcinoma.Mol Med Rep. 2018 Mar;17(3):4237-4244. doi: 10.3892/mmr.2018.8381. Epub 2018 Jan 5. Mol Med Rep. 2018. PMID: 29328455 Free PMC article.
-
Downregulation of microRNA‑21 expression inhibits proliferation, and induces G1 arrest and apoptosis via the PTEN/AKT pathway in SKM‑1 cells.Mol Med Rep. 2018 Sep;18(3):2771-2779. doi: 10.3892/mmr.2018.9255. Epub 2018 Jul 5. Mol Med Rep. 2018. PMID: 30015844 Free PMC article.
-
The Long Non-Coding RNA SNHG1 Attenuates Cell Apoptosis by Regulating miR-195 and BCL2-Like Protein 2 in Human Cardiomyocytes.Cell Physiol Biochem. 2018;50(3):1029-1040. doi: 10.1159/000494514. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. PMID: 30355909
-
MicroRNA-16 Promotes Ovarian Granulosa Cell Proliferation and Suppresses Apoptosis Through Targeting PDCD4 in Polycystic Ovarian Syndrome.Cell Physiol Biochem. 2018;48(2):670-682. doi: 10.1159/000491894. Epub 2018 Jul 19. Cell Physiol Biochem. 2018. PMID: 30025387
-
The Role and Mechanism of MicroRNA 21 in Osteogenesis: An Update.Int J Mol Sci. 2023 Jul 11;24(14):11330. doi: 10.3390/ijms241411330. Int J Mol Sci. 2023. PMID: 37511090 Free PMC article. Review.
Cited by
-
MicroRNA Dysregulation in Epilepsy: From Pathogenetic Involvement to Diagnostic Biomarker and Therapeutic Agent Development.Front Mol Neurosci. 2021 Mar 12;14:650372. doi: 10.3389/fnmol.2021.650372. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33776649 Free PMC article. Review.
-
MicroRNA expression profile by next-generation sequencing in a novel rat model of contrast-induced acute kidney injury.Ann Transl Med. 2019 Apr;7(8):178. doi: 10.21037/atm.2019.04.44. Ann Transl Med. 2019. PMID: 31168459 Free PMC article.
-
miR-21a inhibits decidual cell apoptosis by targeting Pdcd4.Genes Dis. 2019 Oct 5;8(2):171-180. doi: 10.1016/j.gendis.2019.09.013. eCollection 2021 Mar. Genes Dis. 2019. PMID: 33997164 Free PMC article.
-
Microvesicles derived from human umbilical cord mesenchymal stem cells ameliorate renal ischemia-reperfusion injury via delivery of miR-21.Cell Cycle. 2020 Jun;19(11):1285-1297. doi: 10.1080/15384101.2020.1748940. Epub 2020 Apr 24. Cell Cycle. 2020. PMID: 32329653 Free PMC article.
-
Protective Effects of Amlodipine Pretreatment on Contrast-Induced Acute Kidney Injury And Overall Survival In Hypertensive Patients.Front Pharmacol. 2020 Feb 11;11:44. doi: 10.3389/fphar.2020.00044. eCollection 2020. Front Pharmacol. 2020. PMID: 32116719 Free PMC article.
References
-
- Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. - DOI - PMC - PubMed
-
- Perrin T, Descombes E, Cook S. Contrast-induced nephropathy in invasive cardiology. Swiss Med Wkly. 2012;142:w13608. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

