N‑acetylcysteine induces apoptosis via the mitochondria‑dependent pathway but not via endoplasmic reticulum stress in H9c2 cells
- PMID: 28901511
- PMCID: PMC5865795
- DOI: 10.3892/mmr.2017.7442
N‑acetylcysteine induces apoptosis via the mitochondria‑dependent pathway but not via endoplasmic reticulum stress in H9c2 cells
Abstract
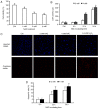
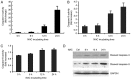
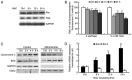
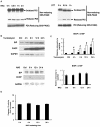
N‑acetylcysteine (NAC), a precursor of glutathione, is a widely used thiol‑containing antioxidant and modulator of the intracellular redox state. Our previous study demonstrated that excess reduced glutathione (GSH) from NAC treatment paradoxically led to a reduction in glutathione redox potential, increased mitochondrial oxidation and caused cytotoxicity at lower reactive oxygen species levels in H9c2 cells. However, no detailed data are available on the molecular mechanisms of NAC‑induced cytotoxicity on H9c2 cells. In the present study, it was demonstrated that NAC‑induced cytotoxicity towards H9c2 cells was associated with apoptosis. The activation of caspase‑9 and ‑3, and cleavage of procaspase‑9 and ‑3, but not of caspase‑8, were involved in NAC‑induced apoptosis. The dissipation of mitochondrial transmembrane potential, release of cytochrome c, translocation of B cell lymphoma‑2 (Bcl‑2)‑associated X protein (Bax) to the mitochondria, and the increased ratio of Bax/Bcl‑2 mRNA indicated that NAC treatment‑induced apoptosis occurred mainly through the mitochondria‑dependent pathway. Redox western blot analysis demonstrated that NAC did not disrupt the highly oxidized environment of the endoplasmic reticulum, which was indicated by maintenance of the oxidized form of protein disulfide isomerase, an essential chaperone in the formation of disulfide bond formation in the endoplasmic reticulum. In addition, no significant changes in the expression of binding immunoglobulin protein or C/EBP homologous protein were apparent in the process of NAC‑induced apoptosis. Taken together, the present study demonstrated for the first time, to the best of our knowledge, that NAC induced apoptosis via the mitochondria‑dependent pathway but not via endoplasmic reticulum stress in H9c2 cells, and the exogenous GSH from NAC did not alter the oxidized milieu of the endoplasmic reticulum.
Figures
Similar articles
-
Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress.Neurol Res. 2019 Jul;41(7):594-608. doi: 10.1080/01616412.2019.1596206. Epub 2019 Apr 11. Neurol Res. 2019. PMID: 30973085
-
Luteolin induces apoptosis through endoplasmic reticulum stress and mitochondrial dysfunction in Neuro-2a mouse neuroblastoma cells.Eur J Pharmacol. 2011 Oct 1;668(1-2):115-26. doi: 10.1016/j.ejphar.2011.06.047. Epub 2011 Jul 8. Eur J Pharmacol. 2011. PMID: 21762691
-
Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction.Biol Pharm Bull. 2010;33(10):1644-9. doi: 10.1248/bpb.33.1644. Biol Pharm Bull. 2010. PMID: 20930370
-
BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity.Free Radic Biol Med. 1999 Nov;27(9-10):945-50. doi: 10.1016/s0891-5849(99)00174-4. Free Radic Biol Med. 1999. PMID: 10569627 Review.
-
Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain.J Inorg Biochem. 2003 Sep 15;97(1):151-4. doi: 10.1016/s0162-0134(03)00258-7. J Inorg Biochem. 2003. PMID: 14507471 Review.
Cited by
-
Optimal Regimen of N-Acetylcysteine on Chromium-Induced Renal Cell Damage.Metabolites. 2019 Aug 28;9(9):172. doi: 10.3390/metabo9090172. Metabolites. 2019. PMID: 31466266 Free PMC article.
-
Intracellular Redox-Modulated Pathways as Targets for Effective Approaches in the Treatment of Viral Infection.Int J Mol Sci. 2021 Mar 30;22(7):3603. doi: 10.3390/ijms22073603. Int J Mol Sci. 2021. PMID: 33808471 Free PMC article. Review.
-
Blockade of p38 MAPK overcomes AML stem cell line KG1a resistance to 5-Fluorouridine and the impact on miRNA profiling.PLoS One. 2022 May 5;17(5):e0267855. doi: 10.1371/journal.pone.0267855. eCollection 2022. PLoS One. 2022. PMID: 35511922 Free PMC article.
-
In Vitro Culture of Vitrified Immature Mouse Testicular Tissue in The Presence of N-acetylcysteine Antioxidant.Int J Fertil Steril. 2025 May 14;19(3):296-304. doi: 10.22074/ijfs.2025.2039641.1749. Int J Fertil Steril. 2025. PMID: 40590290 Free PMC article.
-
Role of Klotho and N-acetylcysteine in Oxidative Stress Associated with the Vitrification of Ovarian Tissue Cytoprotective Function of Klotho in Cryopreservation.Tissue Eng Regen Med. 2023 Jul;20(4):637-646. doi: 10.1007/s13770-023-00556-7. Epub 2023 Jun 23. Tissue Eng Regen Med. 2023. PMID: 37351787 Free PMC article.
References
-
- Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

