A Benefit-Risk Analysis Approach to Capture Regulatory Decision-Making: Multiple Myeloma
- PMID: 28901535
- PMCID: PMC7418461
- DOI: 10.1002/cpt.871
A Benefit-Risk Analysis Approach to Capture Regulatory Decision-Making: Multiple Myeloma
Abstract
Drug regulators around the world make decisions about drug approvability based on qualitative benefit-risk analysis. In this work, a quantitative benefit-risk analysis approach captures regulatory decision-making about new drugs to treat multiple myeloma (MM). MM assessments have been based on endpoints such as time to progression (TTP), progression-free survival (PFS), and objective response rate (ORR) which are different than benefit-risk analysis based on overall survival (OS). Twenty-three FDA decisions on MM drugs submitted to FDA between 2003 and 2016 were identified and analyzed. The benefits and risks were quantified relative to comparators (typically the control arm of the clinical trial) to estimate whether the median benefit-risk was positive or negative. A sensitivity analysis was demonstrated using ixazomib to explore the magnitude of uncertainty. FDA approval decision outcomes were consistent and logical using this benefit-risk framework.
© 2017 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declared no conflicts of interest.
Figures
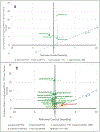

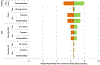
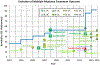
References
-
- Frey P Benefit-Risk Framework (U.S. Food and Drug Administration, CDER, Silver Spring, MD, 2012).
-
- U.S. Food and Drug Administration. Structured Approach to Benefit-Risk Assessment in Drug Regulatory Decision-Making. Washington, DC; 2013.
-
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD; 2011.
-
- Morris S, Rosenblatt M, Orloff J, Lewis-Hall F & Waldstreicher J The PCAST report: impact and implications for the pharmaceutical industry. Clin. Pharmacol. Ther 94, 300–302 (2013). - PubMed
-
- Woodcock J The PCAST Report on Pharmaceutical Innovation: implications for the FDA. Clin. Pharmacol. Ther 94, 297–300 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

