Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture
- PMID: 28901689
- PMCID: PMC8011329
- DOI: 10.1002/jbm.b.33994
Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture
Abstract
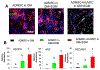
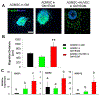
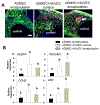
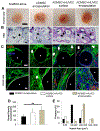
Vascularization is a fundamental prerequisite for large bone construct development and remains one of the main challenges of bone tissue engineering. Our current study presents the combination of 3D printing technique with a hydrogel-based prevascularization strategy to generate prevascularized bone constructs. Human adipose derived mesenchymal stem cells (ADMSC) and human umbilical vein endothelial cells (HUVEC) were encapsulated within our bioactive hydrogels, and the effects of culture conditions on in vitro vascularization were determined. We further generated composite constructs by forming 3D printed polycaprolactone/hydroxyapatite scaffolds coated with cell-laden hydrogels and determined how the co-culture affected vascularization and osteogenesis. It was demonstrated that 3D co-cultured ADMSC-HUVEC generated capillary-like networks within the porous 3D printed scaffold. The co-culture systems promoted in vitro vascularization, but had no significant effects on osteogenesis. The prevascularized constructs were subcutaneously implanted into nude mice to evaluate the in vivo vascularization capacity and the functionality of engineered vessels. The hydrogel systems facilitated microvessel and lumen formation and promoted anastomosis of vascular networks of human origin with host murine vasculature. These findings demonstrate the potential of prevascularized 3D printed scaffolds with anatomical shape for the healing of larger bone defects. © 2017 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 1788-1798, 2018.
Keywords: 3D printing; adipose derived stem cells; bone tissue engineering; human umbilical vein endothelial cells; vascularization.
© 2017 Wiley Periodicals, Inc.
Figures



Similar articles
-
Divergent effects of premineralization and prevascularization on osteogenesis and vascular integration in humanized tissue engineered bone constructs.Acta Biomater. 2025 Jul 1;201:665-683. doi: 10.1016/j.actbio.2025.06.019. Epub 2025 Jun 11. Acta Biomater. 2025. PMID: 40514004
-
Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats.Tissue Eng Part A. 2017 Jun;23(11-12):546-555. doi: 10.1089/ten.tea.2016.0485. Epub 2017 Mar 10. Tissue Eng Part A. 2017. PMID: 28287922 Free PMC article.
-
3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation.Sci Rep. 2017 Jul 17;7(1):5588. doi: 10.1038/s41598-017-05196-1. Sci Rep. 2017. PMID: 28717129 Free PMC article.
-
Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration.Adv Healthc Mater. 2019 May;8(10):e1801433. doi: 10.1002/adhm.201801433. Epub 2019 Apr 2. Adv Healthc Mater. 2019. PMID: 30938094 Review.
-
Engineered Prevascularization for Oral Tissue Grafting: A Systematic Review.Tissue Eng Part B Rev. 2020 Aug;26(4):383-398. doi: 10.1089/ten.TEB.2020.0093. Epub 2020 Aug 7. Tissue Eng Part B Rev. 2020. PMID: 32597330
Cited by
-
Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells.Int J Mol Sci. 2021 Oct 8;22(19):10890. doi: 10.3390/ijms221910890. Int J Mol Sci. 2021. PMID: 34639228 Free PMC article. Review.
-
In vitro dynamic perfusion of prevascularized OECs-DBMs (outgrowth endothelial progenitor cell - demineralized bone matrix) complex fused to recipient vessels in an internal inosculation manner.Bioengineered. 2022 Jun;13(6):14270-14281. doi: 10.1080/21655979.2022.2085560. Bioengineered. 2022. PMID: 35734812 Free PMC article.
-
3D bioprinted white adipose model forin vitrostudy of cancer-associated cachexia induced adipose tissue remodeling.Biofabrication. 2022 May 26;14(3):10.1088/1758-5090/ac6c4b. doi: 10.1088/1758-5090/ac6c4b. Biofabrication. 2022. PMID: 35504266 Free PMC article.
-
Recent Advances on Cell-Based Co-Culture Strategies for Prevascularization in Tissue Engineering.Front Bioeng Biotechnol. 2021 Nov 25;9:745314. doi: 10.3389/fbioe.2021.745314. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34900955 Free PMC article. Review.
-
A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology.Int J Mol Sci. 2023 Feb 21;24(5):4312. doi: 10.3390/ijms24054312. Int J Mol Sci. 2023. PMID: 36901743 Free PMC article. Review.
References
-
- Griffin KS, Davis KM, McKinley TO, Anglen JO, Chu TMG, Boerckel JD, Kacena MA. Evolution of bone grafting: Bone grafts and tissue engineering strategies for vascularized bone regeneration. Clin Rev Bone Miner Metab 2015;13:232–244.
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury 2005;36:S20–27. - PubMed
-
- Verrier S, Alini M, Alsberg E, Buchman SR, Kelly D, Laschke MW, Menger MD, Murphy WL, Stegemann JP, Schütz M, Miclau T, Stoddart MJ, Evans C. Tissue engineering and regenerative approaches to improving the healing of large bone defects. European Cells and Materials 2016;32:87–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

