Simian varicella virus inhibits the interferon gamma signalling pathway
- PMID: 28901902
- PMCID: PMC5845570
- DOI: 10.1099/jgv.0.000925
Simian varicella virus inhibits the interferon gamma signalling pathway
Abstract
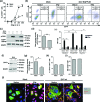
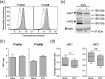
The alphaherpesvirus simian varicella virus (SVV) causes varicella and zoster in nonhuman primates. Herpesviruses evolved elaborate mechanisms to escape host immunity, but the immune evasion strategies employed by SVV remain ill-defined. We analysed whether SVV impairs the cellular response to key antiviral cytokine interferon-γ (IFNγ). SVV infection inhibited the expression of IFNγ-induced genes like C-X-C motif chemokine 10 and interferon regulatory factor 1. Phosphorylation and nuclear translocation of the signal transducer and activator of transcription 1 (STAT1) was blocked in SVV-infected cells, which did not involve cellular and viral phosphatases. SVV infection did not downregulate IFNγ receptor α and β chain expression on the cell surface. Instead, STAT1, Janus tyrosine kinases 1 (JAK1) and JAK2 protein levels were significantly decreased in SVV-infected cells. Collectively, these results demonstrate that SVV targets three proteins in the IFNγ signal transduction pathway to escape the antiviral effects of IFNγ.
Keywords: JAK1; JAK2; STAT1; interferon-γ; simian varicella virus.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
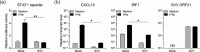
Similar articles
-
The ORF61 Protein Encoded by Simian Varicella Virus and Varicella-Zoster Virus Inhibits NF-κB Signaling by Interfering with IκBα Degradation.J Virol. 2015 Sep;89(17):8687-700. doi: 10.1128/JVI.01149-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085158 Free PMC article.
-
Simian Varicella Virus Infects Enteric Neurons and α4β7 Integrin-Expressing Gut-Tropic T-Cells in Nonhuman Primates.Viruses. 2018 Mar 28;10(4):156. doi: 10.3390/v10040156. Viruses. 2018. PMID: 29597335 Free PMC article.
-
Comparative Analysis of the Simian Varicella Virus and Varicella Zoster Virus Genomes.Viruses. 2022 Apr 19;14(5):844. doi: 10.3390/v14050844. Viruses. 2022. PMID: 35632586 Free PMC article. Review.
-
Attenuation of the adaptive immune response in rhesus macaques infected with simian varicella virus lacking open reading frame 61.J Virol. 2013 Feb;87(4):2151-63. doi: 10.1128/JVI.02369-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221560 Free PMC article.
-
Simian varicella: a model for human varicella-zoster virus infections.Rev Med Virol. 2004 Nov-Dec;14(6):363-81. doi: 10.1002/rmv.437. Rev Med Virol. 2004. PMID: 15386593 Review.
Cited by
-
Insights into the pathogenesis of varicella viruses.Curr Clin Microbiol Rep. 2019 Sep;6(3):156-165. doi: 10.1007/s40588-019-00119-2. Epub 2019 Jul 6. Curr Clin Microbiol Rep. 2019. PMID: 32999816 Free PMC article.
-
LncRNA Nostrill promotes interferon-γ-stimulated gene transcription and facilitates intestinal epithelial cell-intrinsic anti-Cryptosporidium defense.Front Immunol. 2024 Jul 8;15:1397117. doi: 10.3389/fimmu.2024.1397117. eCollection 2024. Front Immunol. 2024. PMID: 39040107 Free PMC article.
-
Manipulation of the Innate Immune Response by Varicella Zoster Virus.Front Immunol. 2020 Jan 24;11:1. doi: 10.3389/fimmu.2020.00001. eCollection 2020. Front Immunol. 2020. PMID: 32038653 Free PMC article. Review.
-
Characteristics of mucin hypersecretion in different inflammatory patterns based on endotypes of chronic rhinosinusitis.Clin Transl Allergy. 2024 Jan;14(1):e12334. doi: 10.1002/clt2.12334. Clin Transl Allergy. 2024. PMID: 38282195 Free PMC article. Review.
-
Alveolar barrier disruption in varicella pneumonia is associated with neutrophil extracellular trap formation.JCI Insight. 2020 Nov 5;5(21):e138900. doi: 10.1172/jci.insight.138900. JCI Insight. 2020. PMID: 33021967 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

