Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer's Disease
- PMID: 28902142
- PMCID: PMC5618614
- DOI: 10.3390/ijms18091965
Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer's Disease
Abstract
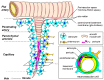
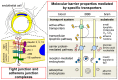
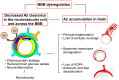
Brain capillary endothelial cells form the blood-brain barrier (BBB), which is covered with basement membranes and is also surrounded by pericytes and astrocyte end-feet in the neurovascular unit. The BBB tightly regulates the molecular exchange between the blood flow and brain parenchyma, thereby regulating the homeostasis of the central nervous system (CNS). Thus, dysfunction of the BBB is likely involved in the pathogenesis of several neurological diseases, including Alzheimer's disease (AD). While amyloid-β (Aβ) deposition and neurofibrillary tangle formation in the brain are central pathological hallmarks in AD, cerebrovascular lesions and BBB alteration have also been shown to frequently coexist. Although further clinical studies should clarify whether BBB disruption is a specific feature of AD pathogenesis, increasing evidence indicates that each component of the neurovascular unit is significantly affected in the presence of AD-related pathologies in animal models and human patients. Conversely, since some portions of Aβ are eliminated along the neurovascular unit and across the BBB, disturbing the pathways may result in exacerbated Aβ accumulation in the brain. Thus, current evidence suggests that BBB dysfunction may causatively and consequently contribute to AD pathogenesis, forming a vicious cycle between brain Aβ accumulation and neurovascular unit impairments during disease progression.
Keywords: amyloid-β; astrocytes; basement membrane; cerebral amyloid angiopathy; endothelial cells; neurovascular unit; pericytes; tight junctions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S., et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Pittman R.N. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. Biota Publishing; Princeton, NJ, USA: 2011. Regulation of Tissue Oxygenation.
-
- Abbas A.K., Lichtman A.H., Pillai S. Cellular and Molecular Immunology. 8th ed. Elsevier Health Sciences; Amsterdam, The Netherlands: 2014. 535p
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

