Optimization and Comparison of Synthetic Procedures for a Group of Triazinyl-Substituted Benzene-Sulfonamide Conjugates with Amino Acids
- PMID: 28902167
- PMCID: PMC6151714
- DOI: 10.3390/molecules22091533
Optimization and Comparison of Synthetic Procedures for a Group of Triazinyl-Substituted Benzene-Sulfonamide Conjugates with Amino Acids
Abstract
Sulfonamides incorporating 1,3,5-triazine moieties can selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII, and XIV over cytosolic isoforms I and II. In the present work, a highly effective synthetic procedure was proposed for this group of potent cancerostatic drugs and compared with previously used methods. The synthesis of triazinyl-substituted benzene-sulfonamide conjugates with amino acids can be easily carried out using sodium carbonate-based water solution as a synthetic medium instead of N,N-Diisopropylethylamine/Dimethylformamide. The benefits of this synthetic procedure include: (i) high selectivity of the creation of disubstituted conjugates; (ii) several times higher yield (≥95%) than that achieved previously; (iii) elimination of organic solvents by the use of an environmental friendly water medium (green chemistry); (iv) simple and fast isolation of the product. The synthesis and resulting products were evaluated using TLC, IR, NMR, and MS methods. The present work demonstrates a significant advantage in providing shortened routes to target structures.
Keywords: 1,3,5-triazine conjugates; amino acids; carboanhydrase inhibitors; sulfonamides; synthesis optimization.
Conflict of interest statement
The authors do not have any conflict of interest concerning the present work.
Figures

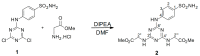
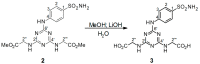
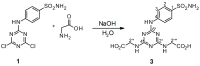


References
-
- Cecchi A., Winum J.Y., Innocenti A., Vullo D., Montero J.L., Scozzafava A., Supuran C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes І, ІІ, and ІX with sulfonamides derived from 4-isothiocyanato-benzolamide. Bioorg. Med. Chem. Lett. 2004;14:5775–5780. doi: 10.1016/j.bmcl.2004.09.062. - DOI - PubMed
-
- Sharma B.K., Pilania P., Singh P., Sharma S., Prabhakar Y.S. CP-MLR directed QSAR study of carbonic anhydrase inhibitors as sulfonamide and sulfamate inhibitors. Cent. Eur. J. Chem. 2009;7:909–922. doi: 10.2478/s11532-009-0073-4. - DOI
-
- Vullo D., Franchi M., Gallori E., Pastorek J., Scozzafava A., Pastorekova S., Supuran C.T. Carbonic anhydrase inhibitors: Inhibition of tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg. Med. Chem. Lett. 2003;13:1005–1009. doi: 10.1016/S0960-894X(03)00091-X. - DOI - PubMed
-
- Carta F., Garaj V., Maresca A., Wagner J., Avvaru B.S., Robbins A.H., Scozzafava A., McKenna R., Supuran C.T. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: Solution and X-ray crystallographic studies. Bioorg. Med. Chem. 2011;19:3105–3119. doi: 10.1016/j.bmc.2011.04.005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

