Longitudinal Effects of Single Hindlimb Radiation Therapy on Bone Strength and Morphology at Local and Contralateral Sites
- PMID: 28902435
- PMCID: PMC5776033
- DOI: 10.1002/jbmr.3289
Longitudinal Effects of Single Hindlimb Radiation Therapy on Bone Strength and Morphology at Local and Contralateral Sites
Abstract
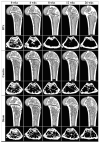
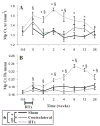
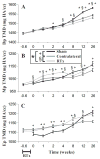
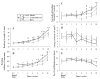
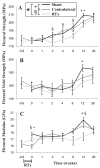
Radiation therapy (RTx) is associated with increased risk for late-onset fragility fractures in bone tissue underlying the radiation field. Bone tissue outside the RTx field is often selected as a "normal" comparator tissue in clinical assessment of fragility fracture risk, but the robustness of this comparison is limited by an incomplete understanding of the systemic effects of local radiotherapy. In this study, a mouse model of limited field irradiation was used to quantify longitudinal changes in local (irradiated) and systemic (non-irradiated) femurs with respect to bone density, morphology, and strength. BALB/cJ mice aged 12 weeks underwent unilateral hindlimb irradiation (4 × 5 Gy) or a sham procedure. Femurs were collected at endpoints of 4 days before treatment and at 0, 1, 2, 4, 8, 12, and 26 weeks post-treatment. Irradiated (RTx), Contralateral (non-RTx), and Sham (non-RTx) femurs were imaged by micro-computed tomography and mechanically tested in three-point bending. In both the RTx and Contralateral non-RTx groups, the longer-term (12- to 26-week) outcomes included trabecular resorption, loss of diaphyseal cortical bone, and decreased bending strength. Contralateral femurs generally followed an intermediate response compared with RTx femurs. Change also varied by anatomic compartment; post-RTx loss of trabecular bone was more profound in the metaphyseal than the epiphyseal compartment, and cortical bone thickness decreased at the mid-diaphysis but increased at the metaphysis. These data demonstrate that changes in bone quantity, density, and architecture occur both locally and systemically after limited field irradiation and vary by anatomic compartment. Furthermore, the severity and persistence of systemic bone damage after limited field irradiation suggest selection of control tissues for assessment of fracture risk or changes in bone density after radiotherapy may be challenging. © 2017 American Society for Bone and Mineral Research.
Keywords: BIOMECHANICS; BONE µ-CT; FRACTURE RISK ASSESSMENT; PRECLINICAL STUDIES; RADIATION-INDUCED BONE DISEASE.
© 2017 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures

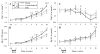



Similar articles
-
Caloric Restriction Diet Attenuates Systemic Bone Fragility after Radiotherapy.Radiat Res. 2024 Nov 1;202(5):765-774. doi: 10.1667/RADE-23-00227.1. Radiat Res. 2024. PMID: 39307529
-
Local irradiation alters bone morphology and increases bone fragility in a mouse model.J Biomech. 2010 Oct 19;43(14):2738-46. doi: 10.1016/j.jbiomech.2010.06.017. Epub 2010 Jul 23. J Biomech. 2010. PMID: 20655052
-
Parathyroid Hormone (1-34) Transiently Protects Against Radiation-Induced Bone Fragility.Calcif Tissue Int. 2016 Jun;98(6):619-30. doi: 10.1007/s00223-016-0111-0. Epub 2016 Feb 4. Calcif Tissue Int. 2016. PMID: 26847434 Free PMC article.
-
The Influence of Cortical Porosity on the Strength of Bone During Growth and Advancing Age.Curr Osteoporos Rep. 2018 Oct;16(5):561-572. doi: 10.1007/s11914-018-0478-0. Curr Osteoporos Rep. 2018. PMID: 30187285 Review.
-
Systematic Review on Multilevel Analysis of Radiation Effects on Bone Microarchitecture.Biomed Res Int. 2022 Jun 6;2022:9890633. doi: 10.1155/2022/9890633. eCollection 2022. Biomed Res Int. 2022. PMID: 35782085 Free PMC article.
Cited by
-
Altered mechanical behavior of demineralized bone following therapeutic radiation.J Orthop Res. 2021 Apr;39(4):750-760. doi: 10.1002/jor.24868. Epub 2020 Oct 6. J Orthop Res. 2021. PMID: 32965711 Free PMC article.
-
Effects of ex vivo ionizing radiation on collagen structure and whole-bone mechanical properties of mouse vertebrae.Bone. 2019 Nov;128:115043. doi: 10.1016/j.bone.2019.115043. Epub 2019 Aug 21. Bone. 2019. PMID: 31445224 Free PMC article.
-
Relative Effects of Radiation-Induced Changes in Bone Mass, Structure, and Tissue Material on Vertebral Strength in a Rat Model.J Bone Miner Res. 2023 Jul;38(7):1032-1042. doi: 10.1002/jbmr.4828. Epub 2023 Jun 4. J Bone Miner Res. 2023. PMID: 37191221 Free PMC article.
-
Postradiation Fractures after Combined Modality Treatment in Extremity Soft Tissue Sarcomas.Sarcoma. 2021 Mar 15;2021:8877567. doi: 10.1155/2021/8877567. eCollection 2021. Sarcoma. 2021. PMID: 33790687 Free PMC article. Review.
-
Influence of radiation exposure pattern on the bone injury and osteoclastogenesis in a rat model.Int J Mol Med. 2019 Dec;44(6):2265-2275. doi: 10.3892/ijmm.2019.4369. Epub 2019 Oct 11. Int J Mol Med. 2019. PMID: 31638191 Free PMC article.
References
-
- Dickie CI, Parent AL, Griffin AM, Fung S, Chung PW, Catton CN, et al. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: relationship to irradiated bone length, volume, tumor location and dose. Int J Radiat Oncol Biol Phys. 2009 Nov 15;75(4):1119–24. Epub 2009/04/14. - PubMed
-
- Cannon CP, Ballo MT, Zagars GK, Mirza AN, Lin PP, Lewis VO, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006 Nov 15;107(10):2455–61. - PubMed
-
- Oh D, Huh SJ, Nam H, Park W, Han Y, Lim do H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1183–8. Epub 2007/10/09. - PubMed
-
- Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004 Sep 1;60(1):265–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

