Rabies screen reveals GPe control of cocaine-triggered plasticity
- PMID: 28902833
- PMCID: PMC6069680
- DOI: 10.1038/nature23888
Rabies screen reveals GPe control of cocaine-triggered plasticity
Abstract
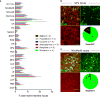
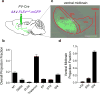
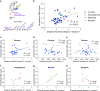
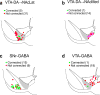
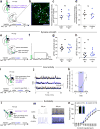
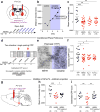
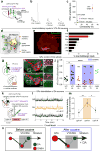
Identification of neural circuit changes that contribute to behavioural plasticity has routinely been conducted on candidate circuits that were preselected on the basis of previous results. Here we present an unbiased method for identifying experience-triggered circuit-level changes in neuronal ensembles in mice. Using rabies virus monosynaptic tracing, we mapped cocaine-induced global changes in inputs onto neurons in the ventral tegmental area. Cocaine increased rabies-labelled inputs from the globus pallidus externus (GPe), a basal ganglia nucleus not previously known to participate in behavioural plasticity triggered by drugs of abuse. We demonstrated that cocaine increased GPe neuron activity, which accounted for the increase in GPe labelling. Inhibition of GPe activity revealed that it contributes to two forms of cocaine-triggered behavioural plasticity, at least in part by disinhibiting dopamine neurons in the ventral tegmental area. These results suggest that rabies-based unbiased screening of changes in input populations can identify previously unappreciated circuit elements that critically support behavioural adaptations.
Figures








References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
-
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

