Reversing behavioural abnormalities in mice exposed to maternal inflammation
- PMID: 28902835
- PMCID: PMC5796433
- DOI: 10.1038/nature23909
Reversing behavioural abnormalities in mice exposed to maternal inflammation
Abstract
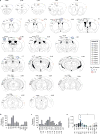
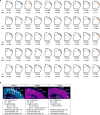
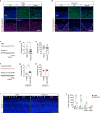
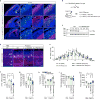
Viral infection during pregnancy is correlated with increased frequency of neurodevelopmental disorders, and this is studied in mice prenatally subjected to maternal immune activation (MIA). We previously showed that maternal T helper 17 cells promote the development of cortical and behavioural abnormalities in MIA-affected offspring. Here we show that cortical abnormalities are preferentially localized to a region encompassing the dysgranular zone of the primary somatosensory cortex (S1DZ). Moreover, activation of pyramidal neurons in this cortical region was sufficient to induce MIA-associated behavioural phenotypes in wild-type animals, whereas reduction in neural activity rescued the behavioural abnormalities in MIA-affected offspring. Sociability and repetitive behavioural phenotypes could be selectively modulated according to the efferent targets of S1DZ. Our work identifies a cortical region primarily, if not exclusively, centred on the S1DZ as the major node of a neural network that mediates behavioural abnormalities observed in offspring exposed to maternal inflammation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures













Comment in
-
Neuroscience: Mum's bacteria linked to baby's behaviour.Nature. 2017 Sep 28;549(7673):466-467. doi: 10.1038/nature24139. Epub 2017 Sep 13. Nature. 2017. PMID: 28902837 Free PMC article.
-
Pregnant mice illuminate risk factors that could lead to autism.Nature. 2017 Sep 13;549(7671):131-132. doi: 10.1038/549131b. Nature. 2017. PMID: 28905926 No abstract available.
-
Brain, Immunity, Gut: "BIG" Links between Pregnancy and Autism.Immunity. 2017 Nov 21;47(5):816-819. doi: 10.1016/j.immuni.2017.10.019. Immunity. 2017. PMID: 29166585 Free PMC article.
References
-
- Atladóttir HO, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. - PubMed
-
- Patterson PH. Immune involvement in schizophrenia and autism:etiology, pathology and animal models. Behav Brain Res. 2009;7:313–321. - PubMed
-
- Atladóttir HO, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. - PubMed
-
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Lekoc Biol. 2006;80:1–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

