Reduced insulin signaling maintains electrical transmission in a neural circuit in aging flies
- PMID: 28902870
- PMCID: PMC5597081
- DOI: 10.1371/journal.pbio.2001655
Reduced insulin signaling maintains electrical transmission in a neural circuit in aging flies
Abstract
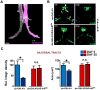
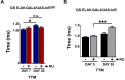
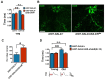
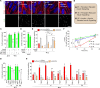
Lowered insulin/insulin-like growth factor (IGF) signaling (IIS) can extend healthy lifespan in worms, flies, and mice, but it can also have adverse effects (the "insulin paradox"). Chronic, moderately lowered IIS rescues age-related decline in neurotransmission through the Drosophila giant fiber system (GFS), a simple escape response neuronal circuit, by increasing targeting of the gap junctional protein innexin shaking-B to gap junctions (GJs). Endosomal recycling of GJs was also stimulated in cultured human cells when IIS was reduced. Furthermore, increasing the activity of the recycling small guanosine triphosphatases (GTPases) Rab4 or Rab11 was sufficient to maintain GJs upon elevated IIS in cultured human cells and in flies, and to rescue age-related loss of GJs and of GFS function. Lowered IIS thus elevates endosomal recycling of GJs in neurons and other cell types, pointing to a cellular mechanism for therapeutic intervention into aging-related neuronal disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152(4):970–81. doi: 10.1016/j.neuroscience.2007.07.014 ; PubMed Central PMCID: PMCPMC2441531. - DOI - PMC - PubMed
-
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17(1):13–8. . - PubMed
-
- Azpurua J, Eaton BA. Neuronal epigenetics and the aging synapse. Front Cell Neurosci. 2015;9:208 doi: 10.3389/fncel.2015.00208 ; PubMed Central PMCID: PMCPMC4444820. - DOI - PMC - PubMed
-
- Dickstein DL, Weaver CM, Luebke JI, Hof PR. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32. doi: 10.1016/j.neuroscience.2012.09.077 ; PubMed Central PMCID: PMCPMC3654095. - DOI - PMC - PubMed
-
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

