TGF-β2-induced ANGPTL4 expression promotes tumor progression and osteoclast differentiation in giant cell tumor of bone
- PMID: 28903395
- PMCID: PMC5589634
- DOI: 10.18632/oncotarget.18629
TGF-β2-induced ANGPTL4 expression promotes tumor progression and osteoclast differentiation in giant cell tumor of bone
Abstract
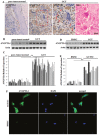
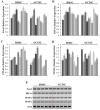
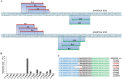
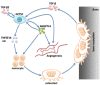
Although emerging studies have implicated that Aiopoietin-like 4 Protein (ANGPTL4) is related to the aggressiveness and metastasis of many tumors, the role of ANGPLT4 in giant cell tumor (GCT) of bone was rarely investigated. The mechanism of ANGPLT4 in tumor-induced osteoclastogenesis still remains unclear. In this study, we first demonstrated that ANGPTL4 was highly expressed in GCT compared to normal tissues, while we showed that TGF-β2 released by osteoclasts induced bone resorption could increase the expression of ANGPTL4 in GCTSCs. By using the luciferase reporter assay, we found that two downstreams of TGF-β2, Smad3 and Smad4, could directly activate the promoter of ANGPTL4, which might explain the mechanism of TGF-β2-induced ANGPLT4 expression. Moreover, knockout of ANGPTL4 by TALENs in GCTSCs inhibited tumor growth, angiogenesis and osteoclastogenesis in GCT in vitro. By using the chick chorio-allantoic membrane (CAM) models, we further showed that inhibition of ANGPTL4 suppressed tumor growth and giant cell formation in vivo. In addition, some new pathways involved in ANGPTL4 application were identified through microarray assay, which may partly explain the mechanism of ANGPTL4 in GCT. Taken together, our study for the first time identified the role of ANGPLT4 in GCT of bone, which may provide a new target for the diagnosis and treatment of GCT.
Keywords: ANGPTL4; angiogenesis; cell proliferation; giant cell tumor of bone; osteoclast differentiation.
Conflict of interest statement
CONFLICTS OF INTEREST All authors declared no conflicts of interest.
Figures




References
-
- Zhou W, Yin H, Wang T, Liu T, Li Z, Yan W, Song D, Chen H, Chen J, Xu W, Yang X, Wu Z, Xiao J. MiR-126-5p regulates osteolysis formation and stromal cell proliferation in giant cell tumor through inhibition of PTHrP. Bone. 2014;66:267–276. - PubMed
-
- Xu W, Li X, Huang W, Wang Y, Han S, Chen S, Xu L, Yang X, Liu T, Xiao J. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Annals of surgical oncology. 2013;20:804–810. - PubMed
-
- Larsson SE, Lorentzon R, Boquist L. Giant-cell tumor of bone. A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. The Journal of bone and joint surgery American volume. 1975;57:167–173. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

