Editor's Highlight: Mechanistic Toxicity Tests Based on an Adverse Outcome Pathway Network for Hepatic Steatosis
- PMID: 28903485
- PMCID: PMC8130650
- DOI: 10.1093/toxsci/kfx121
Editor's Highlight: Mechanistic Toxicity Tests Based on an Adverse Outcome Pathway Network for Hepatic Steatosis
Abstract
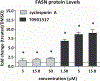
Risk assessors use liver endpoints in rodent toxicology studies to assess the safety of chemical exposures. Yet, rodent endpoints may not accurately reflect human responses. For this reason and others, human-based invitro models are being developed and anchored to adverse outcome pathways to better predict adverse human health outcomes. Here, a networked adverse outcome pathway-guided selection of biology-based assays for lipid uptake, lipid efflux, fatty acid oxidation, and lipid accumulation were developed. These assays were evaluated in a metabolically competent human hepatocyte cell model (HepaRG) exposed to compounds known to cause steatosis (amiodarone, cyclosporine A, and T0901317) or activate lipid metabolism pathways (troglitazone, Wyeth-14,643, and 22(R)-hydroxycholesterol). All of the chemicals activated at least one assay, however, only T0901317 and cyclosporin A dose-dependently increased lipid accumulation. T0901317 and cyclosporin A increased fatty acid uptake, decreased lipid efflux (inferred from apolipoprotein B100 levels), and increased fatty acid synthase protein levels. Using this biologically-based evaluation of key events regulating hepatic lipid levels, we demonstrated dysregulation of compensatory pathways that normally balance hepatic lipid levels. This approach may provide biological plausibility and data needed to increase confidence in linking invitro-based measurements to chemical effects on adverse human health outcomes.
Keywords: adverse outcome pathway; chemical risk assessment; hepatic steatosis; high-throughput toxicity testing; mechanistic toxicology; nonalcoholic fatty liver disease.
Published by Oxford University Press on behalf of the Society of Toxicology 2017. This work is written by US Government employees and is in the public domain in the US.
Figures




References
-
- Angrish MM, Kaiser JP, McQueen CA, and Chorley BN (2016). Tipping the balance: Hepatotoxicity and the 4 apical key events of hepatic steatosis. Toxicol. Sci. 150, 261–268. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. - PubMed
-
- Antherieu S, Rogue A, Fromenty B, Guillouzo A, and Robin MA (2011). Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology 53, 1895–1905. - PubMed
-
- Bergen WG, and Mersmann HJ (2005). Comparative aspects of lipid metabolism: Impact on contemporary research and use of animal models. J. Nutr. 135, 2499–2502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

