The process defines the product: what really matters in biosimilar design and production?
- PMID: 28903544
- PMCID: PMC5850795
- DOI: 10.1093/rheumatology/kex278
The process defines the product: what really matters in biosimilar design and production?
Abstract
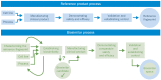
Biologic drugs are highly complex molecules produced by living cells through a multistep manufacturing process. The key characteristics of these molecules, known as critical quality attributes (CQAs), can vary based on post-translational modifications that occur in the cellular environment or during the manufacturing process. The extent of the variation in each of the CQAs must be characterized for the originator molecule and systematically matched as closely as possible by the biosimilar developer to ensure bio-similarity. The close matching of the originator fingerprint is the foundation of the biosimilarity exercise, as the analytical tools designed to measure differences at the molecular level are far more sensitive and specific than tools available to physicians during clinical trials. Biosimilar development, therefore, has a greater focus on preclinical attributes compared with the development of an original biological agent. As changes in CQAs can occur at different stages of the manufacturing process, even small modifications to the process can alter biosimilar attributes beyond the point of similarity and impact clinical effectiveness and safety. The manufacturer's ability to provide consistent production and quality control will greatly influence the acceptance of biosimilars. To this end, preventing drift from the required specifications over time and avoiding the various implications brought by product shortage will enhance biosimilar integration into daily practice. As most prescribers are not familiar with this new drug development paradigm, educational programmes will be needed so that prescribers see biosimilars as fully equivalent, efficacious and safe medicines when compared with originator products.
Keywords: biosimilars; comparability; critical quality attribute; manufacturing; process control; regulatory.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures




References
-
- Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J.. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med 2016;279:14–29. - PubMed
-
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D.. Downstream processing of monoclonal antibodies – application of platform approaches. J Chromatogr B Analyt Technol Biomed Life Sci 2007;848:28–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

