Cellular and molecular mechanisms of viral infection in the human placenta
- PMID: 28903546
- PMCID: PMC7108519
- DOI: 10.1093/femspd/ftx093
Cellular and molecular mechanisms of viral infection in the human placenta
Abstract
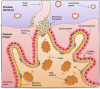
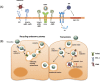
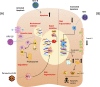
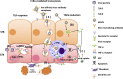
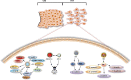
The placenta is a highly specialized organ that is formed during human gestation for conferring protection and generating an optimal microenvironment to maintain the equilibrium between immunological and biochemical factors for fetal development. Diverse pathogens, including viruses, can infect several cellular components of the placenta, such as trophoblasts, syncytiotrophoblasts and other hematopoietic cells. Viral infections during pregnancy have been associated with fetal malformation and pregnancy complications such as preterm labor. In this minireview, we describe the most recent findings regarding virus-host interactions at the placental interface and investigate the mechanisms through which viruses may access trophoblasts and the pathogenic processes involved in viral dissemination at the maternal-fetal interface.
Keywords: maternal–fetal interface; trophoblasts; vertical infection; viral entry; viral pathogenesis; viruses.
© FEMS 2017. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Aldo P, You Y, Szigeti K et al. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am J Reprod Immunol 2016;76:348–57. - PubMed
-
- Aplin JD. The cell biological basis of human implantation. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:757–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

