Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats
- PMID: 28903766
- PMCID: PMC5598033
- DOI: 10.1186/s12974-017-0959-6
Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats
Abstract
Background: The NLRP3 inflammasome is a multiprotein complex that regulates the innate immune inflammatory response by activating caspase-1 and subsequent IL-1β and IL-18. Fluoxetine has been shown to have the anti-inflammatory properties in many disease models. However, the effects and mechanisms of these effects of fluoxetine in early brain injury after subarachnoid hemorrhage (SAH) have not been defined.
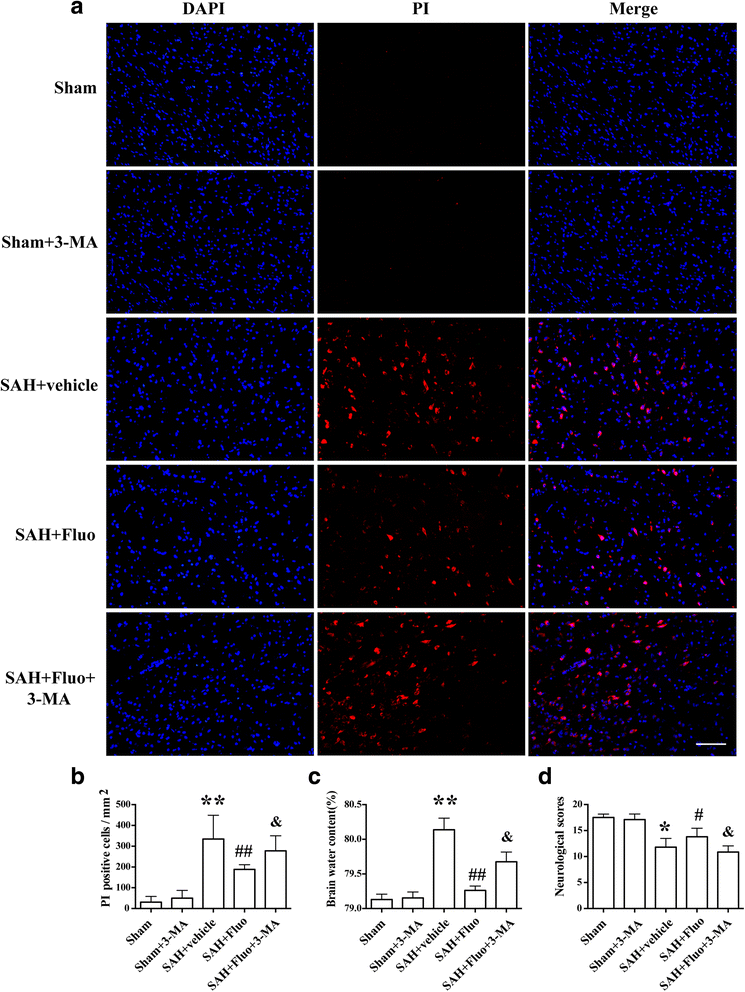
Methods: The SAH model was induced by an endovascular perforation in adult male Sprague-Dawley (SD) rats weighing 300-320 g. N-Ac-Tyr-Val-Ala-Asp-chloromethyl ketone (AC-YVAD-CMK) was injected intraperitoneally (5 mg/kg) 1 h after SAH. Fluoxetine was administered via intravenous route 6 h after SAH. 3-Methyladenine (3-MA) was intracerebroventricularly injected 20 min before SAH. SAH grade, neurological function, brain water content, propidium iodide (PI) staining, western blot, double immunostaining, and transmission electron microscopy were performed.
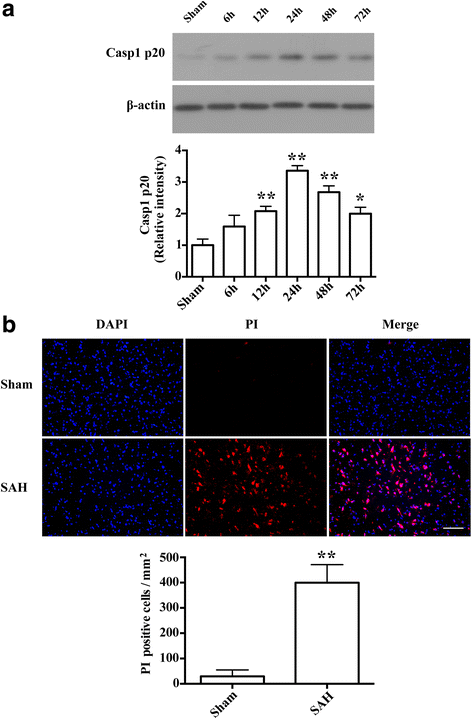
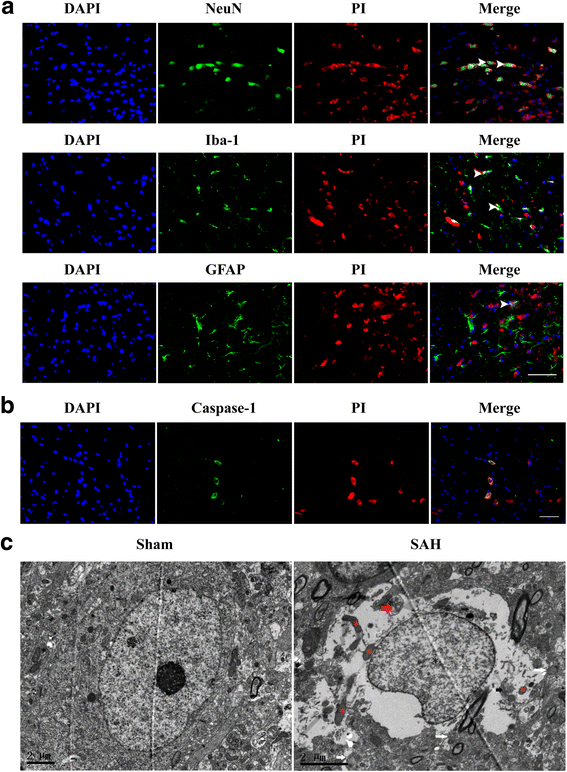
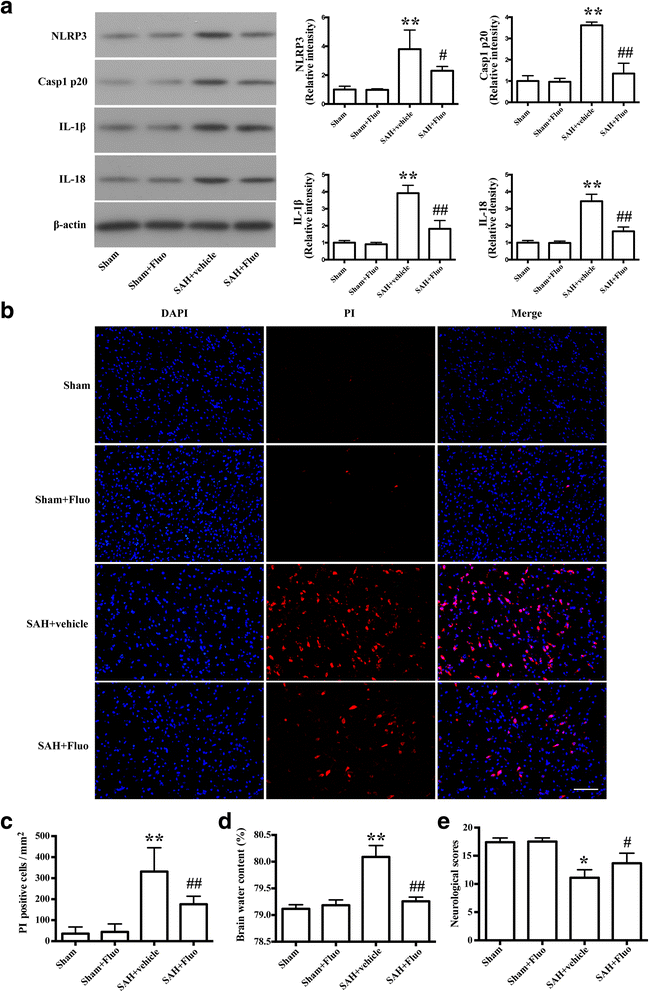
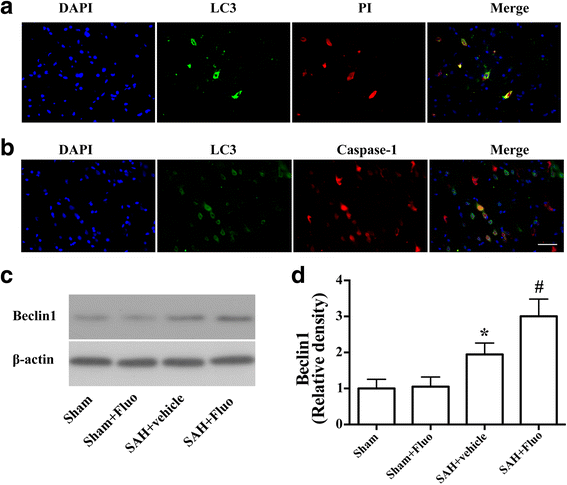
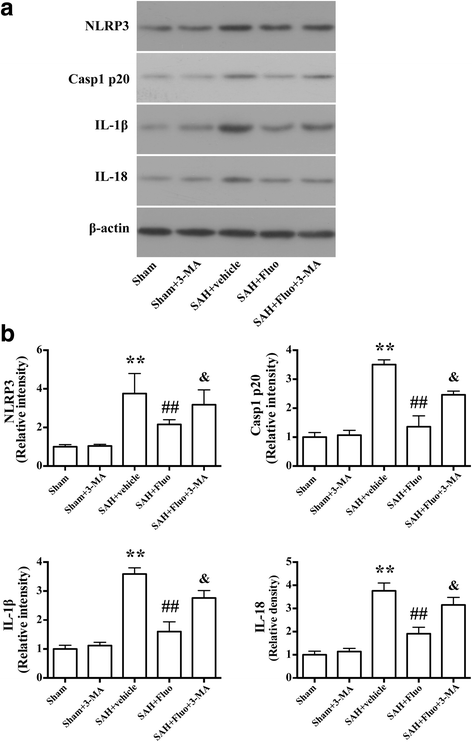
Results: Expression of caspase-1 increased and peaked at 24 h after SAH. Caspase activation was along with the increased necrotic cells, which occurred mainly in neurons. Necrotic cell death of microglia and astrocyte were also found. Administration of AC-YVAD-CMK, a caspase-1 inhibitor, reduced the expression of IL-1β and IL-18 and the number of PI-positive cells, attenuated brain edema, and improved neurological function, which was also observed in fluoxetine-treated rats. Furthermore, fluoxetine treatment significantly decreased the expression of NLRP3 and cleaved caspase-1 and upregulated the expression of beclin-1, a marker for autophagy. Finally, the effects of fluoxetine in NLRP3 inflammasome activation were reversed by additional 3-MA administration.
Conclusions: Together, our present study indicated that NLRP3 inflammasome and caspase-1 activation play a deleterious role in early brain injury and fluoxetine mitigates NLRP3 inflammasome and caspase-1 activation through autophagy activation after SAH, providing a potential therapeutic agent for SAH treatment.
Keywords: Autophagy; Early brain injury; Fluoxetine; Inflammation; NLRP3 inflammasome; Subarachnoid hemorrhage.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. - DOI - PubMed
-
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. - DOI - PubMed
-
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43:1463–1469. doi: 10.1161/STROKEAHA.111.648980. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

