Specific barriers to the conduct of randomised clinical trials on medical devices
- PMID: 28903769
- PMCID: PMC5597993
- DOI: 10.1186/s13063-017-2168-0
Specific barriers to the conduct of randomised clinical trials on medical devices
Abstract
Background: Medical devices play an important role in the diagnosis, prevention, treatment and care of diseases. However, compared to pharmaceuticals, there is no rigorous formal regulation for demonstration of benefits and exclusion of harms to patients. The medical device industry argues that the classical evidence hierarchy cannot be applied for medical devices, as randomised clinical trials are impossible to perform. This article aims to identify the barriers for randomised clinical trials on medical devices.
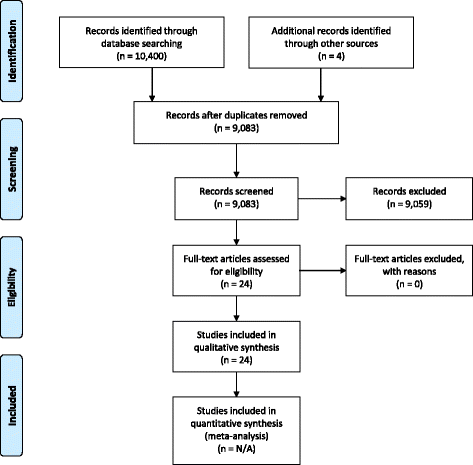
Methods: Systematic literature searches without meta-analysis and internal European Clinical Research Infrastructure Network (ECRIN) communications taking place during face-to-face meetings and telephone conferences from 2013 to 2017 within the context of the ECRIN Integrating Activity (ECRIN-IA) project.
Results: In addition to the barriers that exist for all trials, we identified three major barriers for randomised clinical trials on medical devices, namely: (1) randomisation, including timing of assessment, acceptability, blinding, choice of the comparator group and considerations on the learning curve; (2) difficulties in determining appropriate outcomes; and (3) the lack of scientific advice, regulations and transparency.
Conclusions: The present review offers potential solutions to break down the barriers identified, and argues for applying the randomised clinical trial design when assessing the benefits and harms of medical devices.
Keywords: Assessment; European Clinical Research Infrastructure Network; Evidence-based clinical practice; Evidence-based medicine; Medical devices; Randomised clinical trials; Specific barriers.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors are involved in the ECRIN-IA project.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- European Commission . Growth. Internal Market, Industry, Entrepreneurship and SMEs; Medical Devices. 2016.
-
- Weber S, Haverich A. Bahnbrechende Chirurgische Innovationen in Deutschland: Teil 1: Generierung Medizinischer Evidenz. [Pioneering surgical innovations in Germany: Part 1: generation of medical evidence]. Der Chirurg. Zeitschrift fur alle Gebiete der operativen Medizen. 2016;87(5):423–32. doi: 10.1007/s00104-016-0178-1. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources