Aortic Valve Stenosis Alters Expression of Regional Aortic Wall Shear Stress: New Insights From a 4-Dimensional Flow Magnetic Resonance Imaging Study of 571 Subjects
- PMID: 28903936
- PMCID: PMC5634265
- DOI: 10.1161/JAHA.117.005959
Aortic Valve Stenosis Alters Expression of Regional Aortic Wall Shear Stress: New Insights From a 4-Dimensional Flow Magnetic Resonance Imaging Study of 571 Subjects
Abstract
Background: Wall shear stress (WSS) is a stimulus for vessel wall remodeling. Differences in ascending aorta (AAo) hemodynamics have been reported between bicuspid aortic valve (BAV) and tricuspid aortic valve patients with aortic dilatation, but the confounding impact of aortic valve stenosis (AS) is unknown.
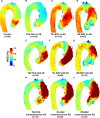
Methods and results: Five hundred seventy-one subjects underwent 4-dimensional flow magnetic resonance imaging in the thoracic aorta (210 right-left BAV cusp fusions, 60 right-noncoronary BAV cusp fusions, 245 tricuspid aortic valve patients with aortic dilatation, and 56 healthy controls). There were 166 of 515 (32%) patients with AS. WSS atlases were created to quantify group-specific WSS patterns in the AAo as a function of AS severity. In BAV patients without AS, the different cusp fusion phenotypes resulted in distinct differences in eccentric WSS elevation: right-left BAV patients exhibited increased WSS by 9% to 34% (P<0.001) at the aortic root and along the entire outer curvature of the AAo whereas right-noncoronary BAV patients showed 30% WSS increase (P<0.001) at the distal portion of the AAo. WSS in tricuspid aortic valve patients with aortic dilatation patients with no AS was significantly reduced by 21% to 33% (P<0.01) in 4 of 6 AAo regions. In all patient groups, mild, moderate, and severe AS resulted in a marked increase in regional WSS (P<0.001). Moderate-to-severe AS further increased WSS magnitude and variability in the AAo. Differences between valve phenotypes were no longer apparent.
Conclusions: AS significantly alters aortic hemodynamics and WSS independent of aortic valve phenotype and over-rides previously described flow patterns associated with BAV and tricuspid aortic valve with aortic dilatation. Severity of AS must be considered when investigating valve-mediated aortopathy.
Keywords: aortic disease; aortic valve; aortic valve stenosis; bicuspid aortic valve; magnetic resonance imaging.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures



References
-
- Roberts WC, Vowels TJ, Ko JM, Filardo G, Hebeler RF Jr, Henry AC, Matter GJ, Hamman BL. Comparison of the structure of the aortic valve and ascending aorta in adults having aortic valve replacement for aortic stenosis versus for pure aortic regurgitation and resection of the ascending aorta for aneurysm. Circulation. 2011;123:896–903. - PubMed
-
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. - PubMed
-
- Fedak PWM, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. - PubMed
-
- Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM III, Enriquez‐Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. - PubMed
-
- Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bossé Y, Limongelli G, Bossone E, Benson DW, Lancellotti P, Isselbacher EM, Enriquez‐Sarano M, Sundt TM III, Pibarot P, Evangelista A, Milewicz DM, Body SC; BAVCon Investigators . Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129:2691–2704. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

