Therapeutic potential of SGN-CD19B, a PBD-based anti-CD19 drug conjugate, for treatment of B-cell malignancies
- PMID: 28903943
- PMCID: PMC5669207
- DOI: 10.1182/blood-2017-04-779389
Therapeutic potential of SGN-CD19B, a PBD-based anti-CD19 drug conjugate, for treatment of B-cell malignancies
Abstract
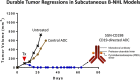
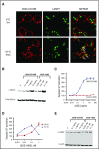
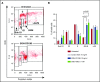
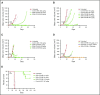
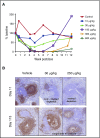
Patients with relapsed/refractory B-cell malignancies such as non-Hodgkin lymphoma (B-NHL) or acute lymphoblastic leukemia have a poor prognosis. Despite measurable clinical activity with new targeted therapies, many patients do not achieve a complete or durable response suggesting an opportunity to improve upon existing therapies. Here we describe SGN-CD19B, a pyrrolobenzodiazepine (PBD)-based anti-CD19 antibody drug conjugate (ADC) being investigated for treatment of B-cell malignancies, which has improved potency compared with other ADCs. CD19-expressing tumor cells rapidly internalize SGN-CD19B, and the released PBD drug induces DNA damage, resulting in G2/M cell cycle arrest and cell death. SGN-CD19B demonstrated activity against a broad panel of malignant B-cell lines and induced durable regressions in mice bearing xenografts derived from these B-cell malignancies. A single dose of SGN-CD19B induced durable regressions at 300 μg/kg (3 μg/kg drug equivalents); combination with rituximab decreased the curative dose to 100 μg/kg (1 μg/kg drug equivalents). These doses are significantly lower than the level of drug required with other ADC payloads. In cynomolgus monkeys, SGN-CD19B effectively depleted CD20+ B lymphocytes in peripheral blood and lymphoid tissues confirming that SGN-CD19B is pharmacodynamically active at well-tolerated doses. In summary, preclinical studies show SGN-CD19B is a highly active ADC, which releases a DNA cross-linking agent rather than a microtubule inhibitor. The distinct mechanism of action, broad potency, and potential to combine with rituximab suggest that SGN-CD19B may offer unique clinical opportunities in B-cell malignancies. A phase 1 clinical trial is in progress to investigate the therapeutic potential of SGN-CD19B in relapsed/refractory B-NHL. This trial was registered at www.clinicaltrials.gov as #NCT02702141.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: All authors are current or former employees of Seattle Genetics, Inc. All studies were paid for by Seattle Genetics, Inc.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. - PubMed
-
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):498-505. - PubMed
-
- Elstrom RL, Martin P, Ostrow K, et al. . Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10(3):192-196. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

