Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance
- PMID: 28904001
- PMCID: PMC6151494
- DOI: 10.1152/physrev.00002.2017
Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance
Abstract
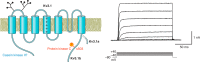
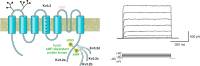
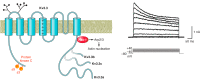
The intrinsic electrical characteristics of different types of neurons are shaped by the K+ channels they express. From among the more than 70 different K+ channel genes expressed in neurons, Kv3 family voltage-dependent K+ channels are uniquely associated with the ability of certain neurons to fire action potentials and to release neurotransmitter at high rates of up to 1,000 Hz. In general, the four Kv3 channels Kv3.1-Kv3.4 share the property of activating and deactivating rapidly at potentials more positive than other channels. Each Kv3 channel gene can generate multiple protein isoforms, which contribute to the high-frequency firing of neurons such as auditory brain stem neurons, fast-spiking GABAergic interneurons, and Purkinje cells of the cerebellum, and to regulation of neurotransmitter release at the terminals of many neurons. The different Kv3 channels have unique expression patterns and biophysical properties and are regulated in different ways by protein kinases. In this review, we cover the function, localization, and modulation of Kv3 channels and describe how levels and properties of the channels are altered by changes in ongoing neuronal activity. We also cover how the protein-protein interaction of these channels with other proteins affects neuronal functions, and how mutations or abnormal regulation of Kv3 channels are associated with neurological disorders such as ataxias, epilepsies, schizophrenia, and Alzheimer's disease.
Copyright © 2017 the American Physiological Society.
Figures

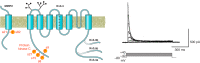




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

