Toward a systems approach to the human cytochrome P450 ensemble: interactions between CYP2D6 and CYP2E1 and their functional consequences
- PMID: 28904078
- PMCID: PMC5798876
- DOI: 10.1042/BCJ20170543
Toward a systems approach to the human cytochrome P450 ensemble: interactions between CYP2D6 and CYP2E1 and their functional consequences
Abstract
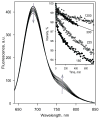
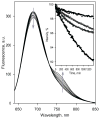
Functional cross-talk among human drug-metabolizing cytochrome P450 through their association is a topic of emerging importance. Here, we studied the interactions of human CYP2D6, a major metabolizer of psychoactive drugs, with one of the most prevalent human P450 enzymes, ethanol-inducible CYP2E1. Detection of P450-P450 interactions was accomplished through luminescence resonance energy transfer between labeled proteins incorporated into human liver microsomes and the microsomes of insect cells containing NADPH-cytochrome P450 reductase. The potential of CYP2D6 to form oligomers in the microsomal membrane is among the highest observed with human cytochrome P450 studied up to date. We also observed the formation of heteromeric complexes of CYP2D6 with CYP2E1 and CYP3A4, and found a significant modulation of these interactions by 3,4-methylenedioxymethylamphetamine, a widespread drug of abuse metabolized by CYP2D6. Our results demonstrate an ample alteration of the catalytic properties of CYP2D6 and CYP2E1 caused by their association. In particular, we demonstrated that preincubation of microsomes containing co-incorporated CYP2D6 and CYP2E1 with CYP2D6-specific substrates resulted in considerable time-dependent activation of CYP2D6, which presumably occurs via a slow substrate-induced reorganization of CYP2E1-CYP2D6 hetero-oligomers. Furthermore, we demonstrated that the formation of heteromeric complexes between CYP2E1 and CYP2D6 affects the stoichiometry of futile cycling and substrate oxidation by CYP2D6 by means of decreasing the electron leakage through the peroxide-generating pathways. Our results further emphasize the role of P450-P450 interactions in regulatory cross-talk in human drug-metabolizing ensemble and suggest a role of interactions of CYP2E1 with CYP2D6 in pharmacologically important instances of alcohol-drug interactions.
Keywords: allosteric regulation; cytochrome P450; drug metabolism; membrane proteins; protein–protein interactions.
© 2017 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Figures




References
-
- Rendic S. Summary of information on human CYP enzymes: Human P450 metabolism data. Drug Metab Rev. 2002;34:83–448. - PubMed
-
- Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Disc. 2005;4:825–833. - PubMed
-
- Gao N, Tian X, Fang Y, Zhou J, Zhang HF, Wen Q, Jia LJ, Gao J, Sun B, Wei JY, Zhang YF, Cui MZ, Qiao HL. Gene polymorphisms and contents of cytochrome P450s have only limited effects on metabolic activities in human liver microsomes. Eur J Pharm Sci. 2016;92:86–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

