Characterization of ion channels and O2 sensitivity in gill neuroepithelial cells of the anoxia-tolerant goldfish (Carassius auratus)
- PMID: 28904098
- PMCID: PMC5712667
- DOI: 10.1152/jn.00237.2017
Characterization of ion channels and O2 sensitivity in gill neuroepithelial cells of the anoxia-tolerant goldfish (Carassius auratus)
Abstract
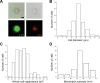
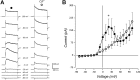
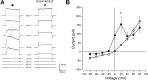
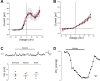
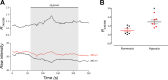
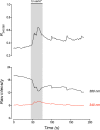
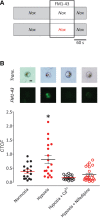
The neuroepithelial cell (NEC) of the fish gill is an important model for O2 sensing in vertebrates; however, a complete picture of the chemosensory mechanisms in NECs is lacking, and O2 chemoreception in vertebrates that are tolerant to anoxia has not yet been explored. Using whole cell patch-clamp recording, we characterized four types of ion channels in NECs isolated from the anoxia-tolerant goldfish. A Ca2+-dependent K+ current (IKCa) peaked at ~20 mV, was potentiated by increased intracellular Ca2+, and was reduced by 100 μM Cd2+ A voltage-dependent inward current in Ba2+ solution, with peak at 0 mV, confirmed the presence of Ca2+ channels. A voltage-dependent K+ current (IKV) was inhibited by 20 mM tetraethylammonium and 5 mM 4-aminopyridine, revealing a background K+ current (IKB) with open rectification. Mean resting membrane potential of -45.2 ± 11.6 mV did not change upon administration of hypoxia (Po2 = 11 mmHg), nor were any of the K+ currents sensitive to changes in Po2 during whole cell recording. By contrast, when the membrane and cytosol were left undisturbed during fura-2 or FM 1-43 imaging experiments, hypoxia increased intracellular Ca2+ concentration and initiated synaptic vesicle activity. 100 μM Cd2+ and 50 μM nifedipine eliminated uptake of FM 1-43. We conclude that Ca2+ influx via L-type Ca2+ channels is correlated with vesicular activity during hypoxic stimulation. In addition, we suggest that expression of IKCa in gill NECs is species specific and, in goldfish, may contribute to an attenuated response to acute hypoxia.NEW & NOTEWORTHY This study provides the first physiological characterization of oxygen chemoreceptors from an anoxia-tolerant vertebrate. Neuroepithelial cells (NECs) from the gills of goldfish displayed L-type Ca2+ channels and three types of K+ channels, one of which was dependent upon intracellular Ca2+ Although membrane currents were not inhibited by hypoxia during patch-clamp recording, this study is the first to show that NECs with an undisturbed cytosol responded to hypoxia with increased intracellular Ca2+ and synaptic vesicle activity.
Keywords: chemoreceptor; goldfish; hypoxia; ion channel; neuroepithelial cell.
Copyright © 2017 the American Physiological Society.
Figures


Similar articles
-
Oxygen sensitivity of gill neuroepithelial cells in the anoxia-tolerant goldfish.Adv Exp Med Biol. 2012;758:167-72. doi: 10.1007/978-94-007-4584-1_23. Adv Exp Med Biol. 2012. PMID: 23080158
-
Neuroepithelial oxygen chemoreceptors of the zebrafish gill.J Physiol. 2004 Nov 1;560(Pt 3):737-52. doi: 10.1113/jphysiol.2004.069294. Epub 2004 Aug 26. J Physiol. 2004. PMID: 15331683 Free PMC article.
-
Heme oxygenase-1 (HO-1) mediated respiratory responses to hypoxia in the goldfish, Carassius auratus.Respir Physiol Neurobiol. 2014 Aug 1;199:1-8. doi: 10.1016/j.resp.2014.04.006. Epub 2014 Apr 26. Respir Physiol Neurobiol. 2014. PMID: 24780551
-
Neuroepithelial cells of the gill and their role in oxygen sensing.Respir Physiol Neurobiol. 2012 Dec 1;184(3):301-8. doi: 10.1016/j.resp.2012.06.024. Epub 2012 Jul 3. Respir Physiol Neurobiol. 2012. PMID: 22772312 Review.
-
Peripheral chemoreceptors in fish: A brief history and a look ahead.Comp Biochem Physiol A Mol Integr Physiol. 2015 Aug;186:27-38. doi: 10.1016/j.cbpa.2014.09.002. Epub 2014 Sep 16. Comp Biochem Physiol A Mol Integr Physiol. 2015. PMID: 25218943 Review.
Cited by
-
The development of the O2-sensing system in an amphibious fish: consequences of variation in environmental O2 levels.J Comp Physiol B. 2021 Jul;191(4):681-699. doi: 10.1007/s00360-021-01379-5. Epub 2021 May 23. J Comp Physiol B. 2021. PMID: 34023926
-
Oxygen chemoreceptor inhibition by dopamine D2 receptors in isolated zebrafish gills.J Physiol. 2025 Apr;603(8):2369-2385. doi: 10.1113/JP287824. Epub 2025 Mar 8. J Physiol. 2025. PMID: 40055972 Free PMC article.
-
Neurochemical Signalling Associated With Gill Oxygen Sensing and Ventilation: A Receptor Focused Mini-Review.Front Physiol. 2022 Jul 13;13:940020. doi: 10.3389/fphys.2022.940020. eCollection 2022. Front Physiol. 2022. PMID: 35910553 Free PMC article. Review.
-
Alterations to cavefish red blood cells provide evidence of adaptation to reduced subterranean oxygen.Sci Rep. 2022 Mar 8;12(1):3735. doi: 10.1038/s41598-022-07619-0. Sci Rep. 2022. PMID: 35260642 Free PMC article.
-
Single-cell transcriptomic analysis of neuroepithelial cells and other cell types of the gills of zebrafish (Danio rerio) exposed to hypoxia.Sci Rep. 2022 Jun 16;12(1):10144. doi: 10.1038/s41598-022-13693-1. Sci Rep. 2022. PMID: 35710785 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

