The superior colliculus and the steering of saccades toward a moving visual target
- PMID: 28904104
- PMCID: PMC5680352
- DOI: 10.1152/jn.00506.2017
The superior colliculus and the steering of saccades toward a moving visual target
Abstract
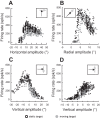
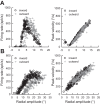
Following the suggestion that a command encoding current target location feeds the oculomotor system during interceptive saccades, we tested the involvement of the deep superior colliculus (dSC). Extracellular activity of 52 saccade-related neurons was recorded in three monkeys while they generated saccades to targets that were static or moving along the preferred axis, away from (outward) or toward (inward) a fixated target with a constant speed (20°/s). Vertical and horizontal motions were tested when possible. Movement field (MF) parameters (boundaries, preferred vector, and firing rate) were estimated after spline fitting of the relation between the average firing rate during the motor burst and saccade amplitude. During radial target motions, the inner MF boundary shifted in the motion direction for some, but not all, neurons. Likewise, for some neurons, the lower boundaries were shifted upward during upward motions and the upper boundaries downward during downward motions. No consistent change was observed during horizontal motions. For some neurons, the preferred vectors were also shifted in the motion direction for outward, upward, and "toward the midline" target motions. The shifts of boundary and preferred vector were not correlated. The burst firing rate was consistently reduced during interceptive saccades. Our study demonstrates an involvement of dSC neurons in steering the interceptive saccade. When observed, the shifts of boundary in the direction of target motion correspond to commands related to past target locations. The absence of shift in the opposite direction implies that dSC activity does not issue predictive commands related to future target location.NEW & NOTEWORTHY The deep superior colliculus is involved in steering the saccade toward the current location of a moving target. During interceptive saccades, the active population consists of a continuum of cells ranging from neurons issuing commands related to past locations of the target to neurons issuing commands related to its current location. The motor burst of collicular neurons does not contain commands related to the future location of a moving target.
Keywords: brain stem; foveation; interception; motion; saccade.
Copyright © 2017 the American Physiological Society.
Figures









Similar articles
-
Activity of neurons in monkey superior colliculus during interrupted saccades.J Neurophysiol. 1996 Jun;75(6):2562-80. doi: 10.1152/jn.1996.75.6.2562. J Neurophysiol. 1996. PMID: 8793764
-
Two-dimensional saccade-related population activity in superior colliculus in monkey.J Neurophysiol. 1998 Aug;80(2):798-817. doi: 10.1152/jn.1998.80.2.798. J Neurophysiol. 1998. PMID: 9705470
-
Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus.J Neurophysiol. 2015 Aug;114(2):879-92. doi: 10.1152/jn.00047.2015. Epub 2015 Jun 10. J Neurophysiol. 2015. PMID: 26063770 Free PMC article.
-
Transforming sensory perceptions into motor commands: evidence from programming of eye movements.Ann N Y Acad Sci. 1997 Dec 19;835:353-62. doi: 10.1111/j.1749-6632.1997.tb48641.x. Ann N Y Acad Sci. 1997. PMID: 9616785 Review.
-
Exploring the superior colliculus in vitro.J Neurophysiol. 2009 Nov;102(5):2581-93. doi: 10.1152/jn.00498.2009. Epub 2009 Aug 26. J Neurophysiol. 2009. PMID: 19710376 Free PMC article. Review.
Cited by
-
Coding of interceptive saccades in parietal cortex of macaque monkeys.Brain Struct Funct. 2021 Nov;226(8):2707-2723. doi: 10.1007/s00429-021-02365-x. Epub 2021 Sep 1. Brain Struct Funct. 2021. PMID: 34468861 Free PMC article.
-
Neurophysiology of visually guided eye movements: critical review and alternative viewpoint.J Neurophysiol. 2018 Dec 1;120(6):3234-3245. doi: 10.1152/jn.00402.2018. Epub 2018 Oct 31. J Neurophysiol. 2018. PMID: 30379628 Free PMC article. Review.
-
Orienting Gaze Toward a Visual Target: Neurophysiological Synthesis with Epistemological Considerations.Vision (Basel). 2025 Jan 14;9(1):6. doi: 10.3390/vision9010006. Vision (Basel). 2025. PMID: 39846622 Free PMC article. Review.
-
Motion Extrapolation for Eye Movements Predicts Perceived Motion-Induced Position Shifts.J Neurosci. 2018 Sep 19;38(38):8243-8250. doi: 10.1523/JNEUROSCI.0736-18.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104339 Free PMC article.
-
Predicted Position Error Triggers Catch-Up Saccades during Sustained Smooth Pursuit.eNeuro. 2020 Jan 15;7(1):ENEURO.0196-18.2019. doi: 10.1523/ENEURO.0196-18.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31862791 Free PMC article.
References
-
- Berthoz A. Simplexity: Simplifying Principles for a Complex World, translated by Weiss G. New Haven, CT: Yale Univ. Press, 2012. doi:10.12987/yale/9780300169348.001.0001. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

