FDA Approval Summary: Daratumumab for Treatment of Multiple Myeloma After One Prior Therapy
- PMID: 28904172
- PMCID: PMC5679834
- DOI: 10.1634/theoncologist.2017-0229
FDA Approval Summary: Daratumumab for Treatment of Multiple Myeloma After One Prior Therapy
Abstract
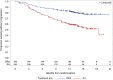
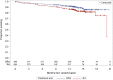
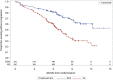
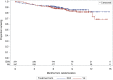
On November 21, 2016, the U.S. Food and Drug Administration granted regular approval to daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least one prior therapy. Approval was based on two randomized, open-label trials in which daratumumab was added to these backbone therapies. The MMY3003 trial demonstrated substantial improvement in progression-free survival (PFS) when daratumumab was added to lenalidomide and dexamethasone compared with lenalidomide and dexamethasone alone. The estimated median PFS had not been reached in the daratumumab arm and was 18.4 months in the control arm (hazard ratio [HR] = 0.37; 95% confidence interval [CI]: 0.27-0.52; p < .0001), representing a 63% reduction in the risk of disease progression or death. Similar results were observed in the MMY3004 trial comparing the combination of daratumumab, bortezomib, and dexamethasone with bortezomib and dexamethasone. The estimated median PFS was not reached in the daratumumab arm and was 7.2 months in the control arm (HR = 0.39; 95% CI: 0.28-0.53; p < .0001), representing a 61% reduction in the risk of disease progression or death. The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3003 were infusion reactions, diarrhea, nausea, fatigue, pyrexia, upper respiratory tract infection, muscle spasm, cough, and dyspnea. The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3004 were infusion reactions, diarrhea, peripheral edema, upper respiratory tract infection, and peripheral sensory neuropathy. Neutropenia and thrombocytopenia have been added to the Warnings and Precautions of the drug label.
Implications for practice: Daratumumab, the first monoclonal antibody targeted against CD38, received U.S. Food and Drug Administration accelerated approval in 2015 based on data from single-agent, single-arm trials that provided response rate information. Results of the MMY3003 and MMY3004 trials established that daratumumab can be combined synergistically with some of the most highly active agents used to treat multiple myeloma, leading to daratumumab's regular approval in 2016. Daratumumab added to lenalidomide and dexamethasone, or bortezomib and dexamethasone, provides a substantial improvement in progression-free survival in previously treated patients with multiple myeloma. These combinations will likely improve the survival outlook for patients with multiple myeloma.
Keywords: Daratumumab; Multiple myeloma.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
EMA Review of Daratumumab for the Treatment of Adult Patients with Multiple Myeloma.Oncologist. 2018 May;23(5):594-602. doi: 10.1634/theoncologist.2017-0328. Epub 2018 Jan 25. Oncologist. 2018. PMID: 29371479 Free PMC article. Clinical Trial.
-
EMA Review of Daratumumab (Darzalex) for the Treatment of Adult Patients Newly Diagnosed with Multiple Myeloma.Oncologist. 2020 Dec;25(12):1067-1074. doi: 10.1002/onco.13554. Epub 2020 Oct 16. Oncologist. 2020. PMID: 33026700 Free PMC article.
-
Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma.N Engl J Med. 2016 Aug 25;375(8):754-66. doi: 10.1056/NEJMoa1606038. N Engl J Med. 2016. PMID: 27557302 Clinical Trial.
-
The European medicines agency review of pomalidomide in combination with low-dose dexamethasone for the treatment of adult patients with multiple myeloma: summary of the scientific assessment of the committee for medicinal products for human use.Oncologist. 2015 Mar;20(3):329-34. doi: 10.1634/theoncologist.2014-0073. Epub 2015 Feb 11. Oncologist. 2015. PMID: 25673103 Free PMC article. Review.
-
Daratumumab: A Review in Relapsed and/or Refractory Multiple Myeloma.Drugs. 2017 Dec;77(18):2013-2024. doi: 10.1007/s40265-017-0837-7. Drugs. 2017. PMID: 29127588 Review.
Cited by
-
Relapsed/Refractory Multiple Myeloma: A Review of Available Therapies and Clinical Scenarios Encountered in Myeloma Relapse.Curr Oncol. 2023 Feb 15;30(2):2322-2347. doi: 10.3390/curroncol30020179. Curr Oncol. 2023. PMID: 36826140 Free PMC article. Review.
-
Novel BCMA-OR-CD38 tandem-dual chimeric antigen receptor T cells robustly control multiple myeloma.Oncoimmunology. 2021 Aug 17;10(1):1959102. doi: 10.1080/2162402X.2021.1959102. eCollection 2021. Oncoimmunology. 2021. PMID: 34434610 Free PMC article.
-
Mechanism research and treatment progress of NAD pathway related molecules in tumor immune microenvironment.Cancer Cell Int. 2022 Jul 30;22(1):242. doi: 10.1186/s12935-022-02664-1. Cancer Cell Int. 2022. PMID: 35906622 Free PMC article. Review.
-
Personalized immunoglobulin aptamers for detection of multiple myeloma minimal residual disease in serum.Commun Biol. 2020 Dec 17;3(1):781. doi: 10.1038/s42003-020-01515-x. Commun Biol. 2020. PMID: 33335255 Free PMC article.
-
Lysosome activable polymeric vorinostat encapsulating PD-L1KD for a combination of HDACi and immunotherapy.Drug Deliv. 2021 Dec;28(1):963-972. doi: 10.1080/10717544.2021.1927246. Drug Deliv. 2021. PMID: 34036867 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017:67:7–30. - PubMed
-
- Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. Available at http://seer.cancer.gov/csr/1975_2013/. Accessed August 2, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

