Dose of Retroviral Infection Determines Induction of Antiviral NK Cell Responses
- PMID: 28904191
- PMCID: PMC5660477
- DOI: 10.1128/JVI.01122-17
Dose of Retroviral Infection Determines Induction of Antiviral NK Cell Responses
Abstract
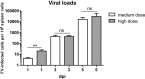
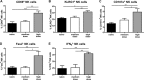
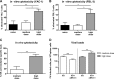
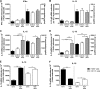
Natural killer (NK) cells are part of the innate immune system and recognize virus-infected cells as well as tumor cells. Conflicting data about the beneficial or even detrimental role of NK cells in different infectious diseases have been described previously. While the type of pathogen strongly influences NK cell functionality, less is known about how the infection dose influences the quality of a NK cell response against retroviruses. In this study, we used the well-established Friend retrovirus (FV) mouse model to investigate the impact of virus dose on the induction of antiviral NK cell functions. High-dose virus inoculation increased initial virus replication compared to that with medium- or low-dose viral challenge and significantly improved NK cell activation. Antiviral NK cell activity, including in vivo cytotoxicity toward infected target cells, was also enhanced by high-dose virus infection. NK cell activation following high-dose viral challenge was likely mediated by activated dendritic cells (DCs) and macrophages and the NK cell-stimulating cytokines interleukin 15 (IL-15) and IL-18. Neutralization of these cytokines decreased NK cell functions and increased viral loads, whereas IL-15 and IL-18 therapy improved NK cell activity. Here we demonstrate that virus dose positively correlates with antiviral NK cell activity and function, which are at least partly driven by IL-15 and IL-18. Our results suggest that NK cell activity may be therapeutically enhanced by administering IL-15 and IL-18 in virus infections that inadequately activate NK cells.IMPORTANCE In infections with retroviruses, like HIV and FV infection of mice, NK cells clearly mediate antiviral activities, but they are usually not sufficient to prevent severe pathology. Here we show that the initial infection dose impacts the induction of an antiviral NK cell response during an acute retroviral infection, which had not investigated before. High-dose infection resulted in a strong NK cell functionality, whereas no antiviral activities were detected after low- or medium-dose infection. Interestingly, DCs and macrophages were highly activated after high-dose FV challenge, which corresponded with increased levels of NK cell-stimulating cytokines IL-15 and IL-18. IL-15 and IL-18 neutralization decreased NK cell functions, whereas IL-15 and IL-18 therapy improved NK cell activity. Here we show the importance of cytokines for NK cell activation in retroviral infections; our findings suggest that immunotherapy combining the well-tolerated cytokines IL-15 and IL-18 might be an interesting approach for antiretroviral treatment.
Keywords: Friend retrovirus; antiviral activity; interleukins; natural killer cells; virus dose.
Copyright © 2017 Littwitz-Salomon et al.
Figures

Similar articles
-
The Cytotoxic Activity of Natural Killer Cells Is Suppressed by IL-10+ Regulatory T Cells During Acute Retroviral Infection.Front Immunol. 2018 Aug 27;9:1947. doi: 10.3389/fimmu.2018.01947. eCollection 2018. Front Immunol. 2018. PMID: 30210499 Free PMC article.
-
Insufficient natural killer cell responses against retroviruses: how to improve NK cell killing of retrovirus-infected cells.Retrovirology. 2016 Nov 8;13(1):77. doi: 10.1186/s12977-016-0311-8. Retrovirology. 2016. PMID: 27821119 Free PMC article. Review.
-
Friend retrovirus infection induces the development of memory-like natural killer cells.Retrovirology. 2018 Oct 6;15(1):68. doi: 10.1186/s12977-018-0450-1. Retrovirology. 2018. PMID: 30292240 Free PMC article.
-
Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection.Retrovirology. 2013 Nov 1;10:127. doi: 10.1186/1742-4690-10-127. Retrovirology. 2013. PMID: 24182203 Free PMC article.
-
Natural killer cells in antiviral defense: function and regulation by innate cytokines.Annu Rev Immunol. 1999;17:189-220. doi: 10.1146/annurev.immunol.17.1.189. Annu Rev Immunol. 1999. PMID: 10358757 Review.
Cited by
-
Iron Metabolism and Immune Regulation.Front Immunol. 2022 Mar 23;13:816282. doi: 10.3389/fimmu.2022.816282. eCollection 2022. Front Immunol. 2022. PMID: 35401569 Free PMC article. Review.
-
Initial Inoculum and the Severity of COVID-19: A Mathematical Modeling Study of the Dose-Response of SARS-CoV-2 Infections.Epidemiologia (Basel). 2020 Oct 21;1(1):5-15. doi: 10.3390/epidemiologia1010003. Epidemiologia (Basel). 2020. PMID: 36417207 Free PMC article.
-
The Cytotoxic Activity of Natural Killer Cells Is Suppressed by IL-10+ Regulatory T Cells During Acute Retroviral Infection.Front Immunol. 2018 Aug 27;9:1947. doi: 10.3389/fimmu.2018.01947. eCollection 2018. Front Immunol. 2018. PMID: 30210499 Free PMC article.
-
Metabolic requirements of NK cells during the acute response against retroviral infection.Nat Commun. 2021 Sep 10;12(1):5376. doi: 10.1038/s41467-021-25715-z. Nat Commun. 2021. PMID: 34508086 Free PMC article.
-
Friend retrovirus studies reveal complex interactions between intrinsic, innate and adaptive immunity.FEMS Microbiol Rev. 2019 Sep 1;43(5):435-456. doi: 10.1093/femsre/fuz012. FEMS Microbiol Rev. 2019. PMID: 31087035 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

