Role of Herpes Simplex Virus 1 γ34.5 in the Regulation of IRF3 Signaling
- PMID: 28904192
- PMCID: PMC5686731
- DOI: 10.1128/JVI.01156-17
Role of Herpes Simplex Virus 1 γ34.5 in the Regulation of IRF3 Signaling
Abstract
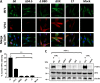
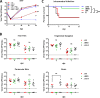
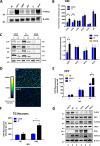
During viral infection, pattern recognition receptors (PRRs) and their associated adaptors recruit TANK-binding kinase 1 (TBK1) to activate interferon regulatory factor 3 (IRF3), resulting in production of type I interferons (IFNs). ICP0 and ICP34.5 are among the proteins encoded by herpes simplex virus 1 (HSV-1) that modulate type I IFN signaling. We constructed a recombinant virus (ΔXX) that lacks amino acids 87 to 106, a portion of the previously described TBK1-binding domain of the γ34.5 gene (D. Verpooten, Y. Ma, S. Hou, Z. Yan, and B. He, J Biol Chem 284:1097-1105, 2009, https://doi.org/10.1074/JBC.M805905200). These 20 residues are outside the γ34.5 beclin1-binding domain (BBD) that interacts with beclin1 and regulates autophagy. Unexpectedly, ΔXX showed no deficit in replication in vivo in a variety of tissues and showed virulence comparable to that of wild-type and marker-rescued viruses following intracerebral infection. ΔXX was fully capable of mediating the dephosphorylation of eIF2α, and the virus was capable of controlling the phosphorylation of IRF3. In contrast, a null mutant in γ34.5 failed to control IRF3 phosphorylation due to an inability of the mutant to sustain expression of ICP0. Our data show that while γ34.5 regulates IRF3 phosphorylation, the TBK1-binding domain itself has no impact on IRF3 phosphorylation or on replication and pathogenesis in mice.IMPORTANCE Interferons (IFNs) are potent activators of a variety of host responses that serve to control virus infections. The Herpesviridae have evolved countermeasures to IFN responses. Herpes simplex virus 1 (HSV-1) encodes the multifunctional neurovirulence protein ICP34.5. In this study, we investigated the biological relevance of the interaction between ICP34.5 and TANK-binding kinase 1 (TBK1), an activator of IFN responses. Here, we establish that although ICP34.5 binds TBK1 under certain conditions through a TBK1-binding domain (TBD), there was no direct impact of the TBD on viral replication or virulence in mice. Furthermore, we showed that activation of IRF3, a substrate of TBK1, was independent of the TBD. Instead, we provided evidence that the ability of ICP34.5 to control IRF3 activation is through its ability to reverse translational shutoff and sustain the expression of other IFN inhibitors encoded by the virus. This work provides new insights into the immunomodulatory functions of ICP34.5.
Keywords: ICP0; ICP34.5; IRF3; RL1; TBK1; herpes simplex virus; innate immunity; interferons; recombinant viruses; viral pathogenesis.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Herpes Simplex Virus Type 1 Interactions with the Interferon System.Int J Mol Sci. 2020 Jul 21;21(14):5150. doi: 10.3390/ijms21145150. Int J Mol Sci. 2020. PMID: 32708188 Free PMC article. Review.
-
Herpes Simplex Virus 1 Serine Protease VP24 Blocks the DNA-Sensing Signal Pathway by Abrogating Activation of Interferon Regulatory Factor 3.J Virol. 2016 May 27;90(12):5824-5829. doi: 10.1128/JVI.00186-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27076640 Free PMC article.
-
Herpes Simplex Virus 1 Inhibits TANK-Binding Kinase 1 through Formation of the Us11-Hsp90 Complex.J Virol. 2018 Jun 29;92(14):e00402-18. doi: 10.1128/JVI.00402-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743370 Free PMC article.
-
The Molecular Mechanism of Herpes Simplex Virus 1 UL31 in Antagonizing the Activity of IFN-β.Microbiol Spectr. 2022 Feb 23;10(1):e0188321. doi: 10.1128/spectrum.01883-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196784 Free PMC article.
-
TANK-binding kinase 1 as a novel therapeutic target for viral diseases.Expert Opin Ther Targets. 2019 May;23(5):437-446. doi: 10.1080/14728222.2019.1601702. Epub 2019 Apr 10. Expert Opin Ther Targets. 2019. PMID: 30932713 Review.
Cited by
-
Herpes Simplex Virus Type 1 Interactions with the Interferon System.Int J Mol Sci. 2020 Jul 21;21(14):5150. doi: 10.3390/ijms21145150. Int J Mol Sci. 2020. PMID: 32708188 Free PMC article. Review.
-
Oncolytic virus: A catalyst for the treatment of gastric cancer.Front Oncol. 2022 Nov 24;12:1017692. doi: 10.3389/fonc.2022.1017692. eCollection 2022. Front Oncol. 2022. PMID: 36505792 Free PMC article. Review.
-
The ICP0 Protein of Herpes Simplex Virus 1 (HSV-1) Downregulates Major Autophagy Adaptor Proteins Sequestosome 1 and Optineurin during the Early Stages of HSV-1 Infection.J Virol. 2019 Oct 15;93(21):e01258-19. doi: 10.1128/JVI.01258-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375597 Free PMC article.
-
Foot-and-mouth disease virus VP1 degrades YTHDF2 through autophagy to regulate IRF3 activity for viral replication.Autophagy. 2024 Jul;20(7):1597-1615. doi: 10.1080/15548627.2024.2330105. Epub 2024 Mar 22. Autophagy. 2024. PMID: 38516932 Free PMC article.
-
African Swine Fever Virus E120R Protein Inhibits Interferon Beta Production by Interacting with IRF3 To Block Its Activation.J Virol. 2021 Aug 25;95(18):e0082421. doi: 10.1128/JVI.00824-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190598 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

