A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal
- PMID: 28904206
- PMCID: PMC5662265
- DOI: 10.1091/mbc.E17-06-0421
A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal
Abstract
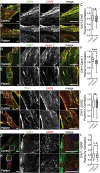
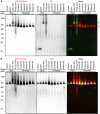
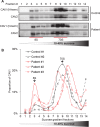
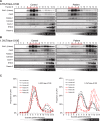
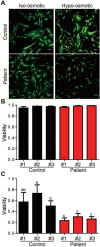
Caveolin-1 (CAV1) is an essential component of caveolae and is implicated in numerous physiological processes. Recent studies have identified heterozygous mutations in the CAV1 gene in patients with pulmonary arterial hypertension (PAH), but the mechanisms by which these mutations impact caveolae assembly and contribute to disease remain unclear. To address this question, we examined the consequences of a familial PAH-associated frameshift mutation in CAV1, P158PfsX22, on caveolae assembly and function. We show that C-terminus of the CAV1 P158 protein contains a functional ER-retention signal that inhibits ER exit and caveolae formation and accelerates CAV1 turnover in Cav1-/- MEFs. Moreover, when coexpressed with wild-type (WT) CAV1 in Cav1-/- MEFs, CAV1-P158 functions as a dominant negative by partially disrupting WT CAV1 trafficking. In patient skin fibroblasts, CAV1 and caveolar accessory protein levels are reduced, fewer caveolae are observed, and CAV1 complexes exhibit biochemical abnormalities. Patient fibroblasts also exhibit decreased resistance to a hypo-osmotic challenge, suggesting the function of caveolae as membrane reservoir is compromised. We conclude that the P158PfsX22 frameshift introduces a gain of function that gives rise to a dominant negative form of CAV1, defining a new mechanism by which disease-associated mutations in CAV1 impair caveolae assembly.
© 2017 Copeland, Han, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



Similar articles
-
Characterization of a caveolin-1 mutation associated with both pulmonary arterial hypertension and congenital generalized lipodystrophy.Traffic. 2016 Dec;17(12):1297-1312. doi: 10.1111/tra.12452. Epub 2016 Nov 2. Traffic. 2016. PMID: 27717241 Free PMC article.
-
Aberrant caveolin-1-mediated Smad signaling and proliferation identified by analysis of adenine 474 deletion mutation (c.474delA) in patient fibroblasts: a new perspective on the mechanism of pulmonary hypertension.Mol Biol Cell. 2017 May 1;28(9):1177-1185. doi: 10.1091/mbc.E16-11-0790. Mol Biol Cell. 2017. PMID: 28468941 Free PMC article.
-
Caveolar domain organization and trafficking is regulated by Abl kinases and mDia1.J Cell Sci. 2012 Jul 1;125(Pt 13):3097-113. doi: 10.1242/jcs.090134. Epub 2012 Mar 27. J Cell Sci. 2012. PMID: 22454521
-
Caveolar and non-Caveolar Caveolin-1 in ocular homeostasis and disease.Prog Retin Eye Res. 2022 Nov;91:101094. doi: 10.1016/j.preteyeres.2022.101094. Epub 2022 Jun 18. Prog Retin Eye Res. 2022. PMID: 35729002 Free PMC article. Review.
-
Caveolae and cancer: A new mechanical perspective.Biomed J. 2015 Sep-Oct;38(5):367-79. doi: 10.4103/2319-4170.164229. Biomed J. 2015. PMID: 26345539 Review.
Cited by
-
Molecular genetic framework underlying pulmonary arterial hypertension.Nat Rev Cardiol. 2020 Feb;17(2):85-95. doi: 10.1038/s41569-019-0242-x. Epub 2019 Aug 12. Nat Rev Cardiol. 2020. PMID: 31406341 Review.
-
Early proteostasis of caveolins synchronizes trafficking, degradation, and oligomerization to prevent toxic aggregation.J Cell Biol. 2023 Sep 4;222(9):e202204020. doi: 10.1083/jcb.202204020. Epub 2023 Aug 1. J Cell Biol. 2023. PMID: 37526691 Free PMC article.
-
Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix.Cells. 2023 Mar 20;12(6):942. doi: 10.3390/cells12060942. Cells. 2023. PMID: 36980283 Free PMC article. Review.
-
Defining the clinical validity of genes reported to cause pulmonary arterial hypertension.Genet Med. 2023 Nov;25(11):100925. doi: 10.1016/j.gim.2023.100925. Epub 2023 Jul 5. Genet Med. 2023. PMID: 37422716 Free PMC article.
-
RNF213 Acts as a Molecular Switch for Cav-1 Ubiquitination and Phosphorylation in Human Cells.Cells. 2025 May 25;14(11):775. doi: 10.3390/cells14110775. Cells. 2025. PMID: 40497951 Free PMC article.
References
-
- Ariotti N, Parton RG. SnapShot: caveolae, caveolins, and cavins. Cell. 2013;154:704–704 e701. - PubMed
-
- Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliott WM, Szulcek R, Bogaard HJ, et al. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ. 2013;3:816–830. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

