After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination
- PMID: 28904214
- PMCID: PMC5597985
- DOI: 10.1523/JNEUROSCI.1453-17.2017
After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination
Abstract
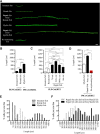
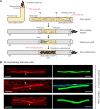
There is consensus that, distal to peripheral nerve injury, myelin and Remak cells reorganize to form cellular columns, Bungner's bands, which are indispensable for regeneration. However, knowledge of the structure of these regeneration tracks has not advanced for decades and the structure of the cells that form them, denervated or repair Schwann cells, remains obscure. Furthermore, the origin of these cells from myelin and Remak cells and their ability to give rise to myelin cells after regeneration has not been demonstrated directly, although these conversions are believed to be central to nerve repair. Using genetic lineage-tracing and scanning-block face electron microscopy, we show that injury of sciatic nerves from mice of either sex triggers extensive and unexpected Schwann cell elongation and branching to form long, parallel processes. Repair cells are 2- to 3-fold longer than myelin and Remak cells and 7- to 10-fold longer than immature Schwann cells. Remarkably, when repair cells transit back to myelinating cells, they shorten ∼7-fold to generate the typically short internodes of regenerated nerves. The present experiments define novel morphological transitions in injured nerves and show that repair Schwann cells have a cell-type-specific structure that differentiates them from other cells in the Schwann cell lineage. They also provide the first direct evidence using genetic lineage tracing for two basic assumptions in Schwann cell biology: that myelin and Remak cells generate the elongated cells that build Bungner bands in injured nerves and that such cells can transform to myelin cells after regeneration.SIGNIFICANCE STATEMENT After injury to peripheral nerves, the myelin and Remak Schwann cells distal to the injury site reorganize and modify their properties to form cells that support the survival of injured neurons, promote axon growth, remove myelin-associated growth inhibitors, and guide regenerating axons to their targets. We show that the generation of these repair-supportive Schwann cells involves an extensive cellular elongation and branching, often to form long, parallel processes. This generates a distinctive repair cell morphology that is favorable for the formation of the regeneration tracks that are essential for nerve repair. Remyelination, conversely, involves a striking cell shortening to form the typical short myelin cells of regenerated nerves. We also provide evidence for direct lineage relationships between: (1) repair cells and myelin and Remak cells of uninjured nerves and (2) remyelinating cells in regenerated nerves.
Keywords: PNS; Schwann cell; injury; nerve; regeneration; remyelination.
Copyright © 2017 Gomez-Sanchez et al.
Figures







References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647. 10.1016/j.neuron.2012.06.021 - DOI - PMC - PubMed
-
- Atanasoski S, Boentert M, De Ventura L, Pohl H, Baranek C, Beier K, Young P, Barbacid M, Suter U (2008) Postnatal Schwann cell proliferation but not myelination is strictly and uniquely dependent on cyclin-dependent kinase 4 (cdk4). Mol Cell Neurosci 37:519–527. 10.1016/j.mcn.2007.11.005 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
