Polyphenols journey through blood-brain barrier towards neuronal protection
- PMID: 28904352
- PMCID: PMC5597593
- DOI: 10.1038/s41598-017-11512-6
Polyphenols journey through blood-brain barrier towards neuronal protection
Erratum in
-
Correction to: Polyphenols journey through blood-brain barrier towards neuronal protection.Sci Rep. 2021 Aug 18;11(1):17112. doi: 10.1038/s41598-021-96179-w. Sci Rep. 2021. PMID: 34408218 Free PMC article. No abstract available.
Abstract
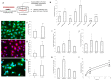
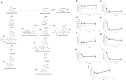
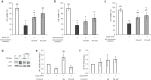
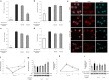
Age-related complications such as neurodegenerative disorders are increasing and remain cureless. The possibility of altering the progression or the development of these multifactorial diseases through diet is an emerging and attractive approach with increasing experimental support. We examined the potential of known bioavailable phenolic sulfates, arising from colonic metabolism of berries, to influence hallmarks of neurodegenerative processes. In silico predictions and in vitro transport studies across blood-brain barrier (BBB) endothelial cells, at circulating concentrations, provided evidence for differential transport, likely related to chemical structure. Moreover, endothelial metabolism of these phenolic sulfates produced a plethora of novel chemical entities with further potential bioactivies. Pre-conditioning with phenolic sulfates improved cellular responses to oxidative, excitotoxicity and inflammatory injuries and this attenuation of neuroinflammation was achieved via modulation of NF-κB pathway. Our results support the hypothesis that these small molecules, derived from dietary (poly)phenols may cross the BBB, reach brain cells, modulate microglia-mediated inflammation and exert neuroprotective effects, with potential for alleviation of neurodegenerative diseases.
Conflict of interest statement
A.F. is employee of Tecnimede – Sociedade Técnico Medicinal, S.A. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
Similar articles
-
Blood-brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: an in vitro study.Eur J Nutr. 2019 Feb;58(1):113-130. doi: 10.1007/s00394-017-1576-y. Epub 2017 Nov 18. Eur J Nutr. 2019. PMID: 29151137
-
Unlocking the potential of low-molecular-weight (Poly)phenol metabolites: Protectors at the blood-brain barrier frontier.Neurochem Int. 2024 Oct;179:105836. doi: 10.1016/j.neuint.2024.105836. Epub 2024 Aug 14. Neurochem Int. 2024. PMID: 39151552 Review.
-
5-(Hydroxyphenyl)-γ-Valerolactone-Sulfate, a Key Microbial Metabolite of Flavan-3-ols, Is Able to Reach the Brain: Evidence from Different in Silico, In Vitro and In Vivo Experimental Models.Nutrients. 2019 Nov 5;11(11):2678. doi: 10.3390/nu11112678. Nutrients. 2019. PMID: 31694297 Free PMC article.
-
Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities.Oxid Med Cell Longev. 2022 Feb 21;2022:5288698. doi: 10.1155/2022/5288698. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237381 Free PMC article. Review.
-
Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation.Ageing Res Rev. 2017 Jul;36:11-19. doi: 10.1016/j.arr.2017.02.004. Epub 2017 Feb 21. Ageing Res Rev. 2017. PMID: 28235660 Review.
Cited by
-
Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components.Molecules. 2023 Feb 24;28(5):2151. doi: 10.3390/molecules28052151. Molecules. 2023. PMID: 36903395 Free PMC article. Review.
-
Cocoa Extract Provides Protection against 6-OHDA Toxicity in SH-SY5Y Dopaminergic Neurons by Targeting PERK.Biomedicines. 2022 Aug 18;10(8):2009. doi: 10.3390/biomedicines10082009. Biomedicines. 2022. PMID: 36009556 Free PMC article.
-
4-Ethylphenol-fluxes, metabolism and excretion of a gut microbiome derived neuromodulator implicated in autism.Front Mol Biosci. 2023 Oct 12;10:1267754. doi: 10.3389/fmolb.2023.1267754. eCollection 2023. Front Mol Biosci. 2023. PMID: 37900921 Free PMC article. Review.
-
The Flavonoid Agathisflavone Attenuates Glia Activation After Mechanical Injury of Cortical Tissue and Negatively Regulates Both NRLP3 and IL-1β Expression.Int J Mol Sci. 2025 Feb 1;26(3):1275. doi: 10.3390/ijms26031275. Int J Mol Sci. 2025. PMID: 39941042 Free PMC article.
-
Pharmacological Potential of Flavonoids against Neurotropic Viruses.Pharmaceuticals (Basel). 2022 Sep 15;15(9):1149. doi: 10.3390/ph15091149. Pharmaceuticals (Basel). 2022. PMID: 36145370 Free PMC article. Review.
References
-
- Anonymous World report on ageing and health - World Health Organization edited by World Health Organization WHO Press (Geneva, 2015).
-
- Figueira, I. et al. Interventions for age-related diseases: Shifting the paradigm. Mech. Ageing Dev (2016). - PubMed
-
- Rodriguez-Mateos A, et al. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013;98(5):1179–1191. doi: 10.3945/ajcn.113.066639. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

