Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management
- PMID: 28904447
- PMCID: PMC5586110
- DOI: 10.4103/aian.AIAN_239_17
Levodopa-induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management
Abstract
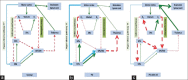
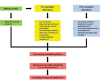
Levodopa-induced dyskinesia (LID) is commonly seen in Parkinson's disease patients treated with levodopa. This side effect is usually encountered after long duration of treatment, but occasionally, this may be seen even after few days or months of treatment. LID is broadly classified as peak-dose dyskinesia, wearing-off or off-period dyskinesia, and diphasic dyskinesia. Pathogenesis of LID is complex, and different neurotransmitters such as dopamine, glutamine, adenosine, and gamma-aminobutyric acid play important role altering the normal physiology of direct and indirect pathway of cortico-basal ganglia-thalamic loop responsible for fine motor control. Treatment of LID requires careful history taking and clinical examination to find the type of dyskinesia as different approach is required for different types. Changes in dopaminergic medication including continuous dopaminergic stimulation are very helpful in the management of peak-dose dyskinesia. Different types of surgical approaches including unilateral pallidotomy and deep brain stimulation have given very good result in patients, who cannot be managed by medications alone. The surgical management of LID is dealt with in detail in another review in this series.
Keywords: Dopamine; Parkinson's disease; dyskinesia; levodopa.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276:374–9. - PubMed
-
- Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with parkinson's disease: Filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–17. - PubMed
-
- Vijayakumar D, Jankovic J. Drug-induced dyskinesia, part 1: Treatment of levodopa-induced dyskinesia. Drugs. 2016;76:759–77. - PubMed
-
- Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in parkinson's disease: Clinical and pharmacological classification. Mov Disord. 1992;7:117–24. - PubMed
-
- Hametner E, Seppi K, Poewe W. The clinical spectrum of levodopa-induced motor complications. J Neurol. 2010;257(Suppl 2):S268–75. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

