Oxidative dehydrogenation of C-C and C-N bonds: A convenient approach to access diverse (dihydro)heteroaromatic compounds
- PMID: 28904611
- PMCID: PMC5564259
- DOI: 10.3762/bjoc.13.162
Oxidative dehydrogenation of C-C and C-N bonds: A convenient approach to access diverse (dihydro)heteroaromatic compounds
Abstract
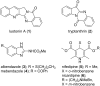
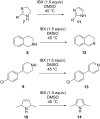
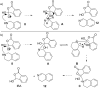
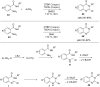
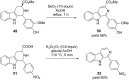
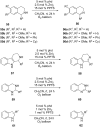
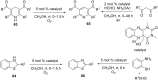
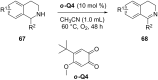
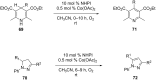
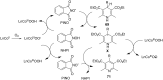
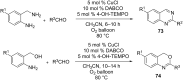
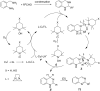
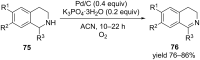
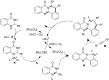
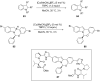
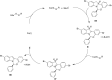
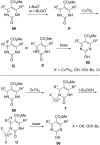
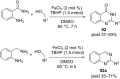
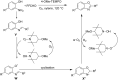
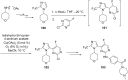
Nitrogen heteroarenes form an important class of compounds which can be found in natural products, synthetic drugs, building blocks etc. Among the diverse strategies that were developed for the synthesis of nitrogen heterocycles, oxidative dehydrogenation is extremely effective. This review discusses various oxidative dehydrogenation strategies of C-C and C-N bonds to generate nitrogen heteroarenes from their corresponding heterocyclic substrates. The strategies are categorized under stoichiometric and catalytic usage of reagents that facilitate such transformations. The application of these strategies in the synthesis of nitrogen heteroarene natural products and synthetic drug intermediates are also discussed. We hope this review will arouse sufficient interest among the scientific community to further advance the application of oxidative dehydrogenation in the synthesis of nitrogen heteroarenes.
Keywords: aerobic oxidation; bioinspired Flavin mimics; nitrogen heteroarenes; organo catalytic; oxidative dehydrogenation.
Figures
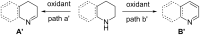
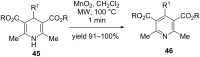


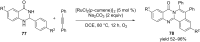

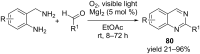
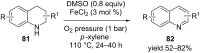

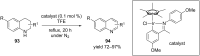

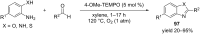
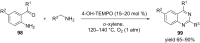
Similar articles
-
Nitrogen-Doped Carbon as a Highly Active Metal-Free Catalyst for the Selective Oxidative Dehydrogenation of N-Heterocycles.ChemSusChem. 2022 Aug 5;15(15):e202200753. doi: 10.1002/cssc.202200753. Epub 2022 Jun 13. ChemSusChem. 2022. PMID: 35504842
-
Recent advances in the synthesis of N-heteroarenes via catalytic dehydrogenation of N-heterocycles.Chem Commun (Camb). 2021 Dec 7;57(97):13042-13058. doi: 10.1039/d1cc04919d. Chem Commun (Camb). 2021. PMID: 34781335 Review.
-
Dehydrogenation of N-Heterocycles by Superoxide Ion Generated through Single-Electron Transfer.Chemistry. 2018 Feb 9;24(9):2065-2069. doi: 10.1002/chem.201705202. Epub 2018 Jan 17. Chemistry. 2018. PMID: 29210122
-
Organo-Photoredox Catalyzed Oxidative Dehydrogenation of N-Heterocycles.Chemistry. 2017 Oct 12;23(57):14167-14172. doi: 10.1002/chem.201703642. Epub 2017 Sep 18. Chemistry. 2017. PMID: 28805268
-
DDQ as a versatile and easily recyclable oxidant: a systematic review.RSC Adv. 2021 Sep 8;11(47):29826-29858. doi: 10.1039/d1ra04575j. eCollection 2021 Sep 1. RSC Adv. 2021. PMID: 35479576 Free PMC article. Review.
Cited by
-
Oxone promoted dehydrogenative Povarov cyclization of N-aryl glycine derivatives: an approach towards quinoline fused lactones and lactams.RSC Adv. 2019 Sep 25;9(52):30277-30291. doi: 10.1039/c9ra06212b. eCollection 2019 Sep 23. RSC Adv. 2019. PMID: 35530246 Free PMC article.
-
Mechanistic insights into excited-state palladium catalysis for C-S bond formations and dehydrogenative sulfonylation of amines.Nat Commun. 2023 Oct 19;14(1):6622. doi: 10.1038/s41467-023-42392-2. Nat Commun. 2023. PMID: 37857662 Free PMC article.
-
Synthesis of N-aryl amines enabled by photocatalytic dehydrogenation.Chem Sci. 2020 Dec 8;12(5):1915-1923. doi: 10.1039/d0sc04890a. Chem Sci. 2020. PMID: 34163955 Free PMC article.
References
-
- Quin L D, Tyrell J A, editors. Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals. 1st ed. Hoboken: John Wiley & Sons; 2010.
-
- Ritchie T J, Macdonald S J F, Peace S, Pickett S D, Luscombe C N. Med Chem Commun. 2012;3:1062–1069. doi: 10.1039/C2MD20111A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
