18-Hydroxydolabella-3,7-diene synthase - a diterpene synthase from Chitinophaga pinensis
- PMID: 28904620
- PMCID: PMC5588592
- DOI: 10.3762/bjoc.13.171
18-Hydroxydolabella-3,7-diene synthase - a diterpene synthase from Chitinophaga pinensis
Abstract
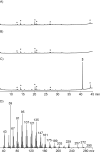
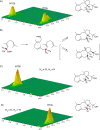
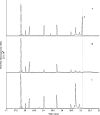
The product obtained in vitro from a diterpene synthase encoded in the genome of the bacterium Chitinophaga pinensis, an enzyme previously reported to have germacrene A synthase activity during heterologous expression in Escherichia coli, was identified by extensive NMR-spectroscopic methods as 18-hydroxydolabella-3,7-diene. The absolute configuration of this diterpene alcohol and the stereochemical course of the terpene synthase reaction were addressed by isotopic labelling experiments. Heterologous expression of the diterpene synthase in Nicotiana benthamiana resulted in the production of 18-hydroxydolabella-3,7-diene also in planta, while the results from the heterologous expression in E. coli were shown to be reproducible, revealing that the expression of one and the same terpene synthase in different heterologous hosts may yield different terpene products.
Keywords: Chitinophaga pinensis; Nicotiana benthamiana; biosynthesis; structure elucidation; terpenes.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
