Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need?
- PMID: 28904804
- PMCID: PMC5590167
- DOI: 10.1186/s40661-017-0050-0
Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need?
Abstract
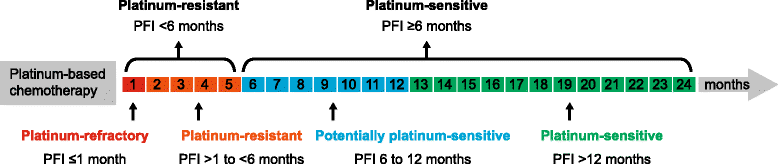
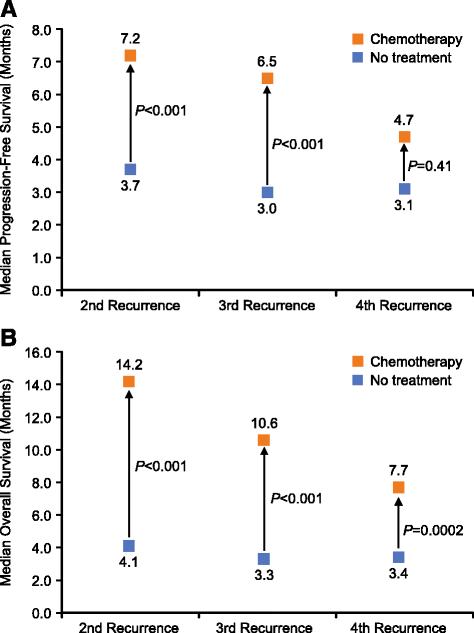
Background: Therapy for advanced epithelial ovarian cancer (OC) includes first line platinum/taxane-containing chemotherapy and re-treatment with platinum-containing regimens for disease recurrence in patients likely to respond again. Single-agent, non-platinum, cytotoxic agents are commonly used to treat patients resistant to platinum retreatment, but these agents are associated with dose-limiting toxicities and response rates below 20%.
Main body: Recent advances have led to novel targeted treatments for recurrent OC that offer opportunities to improve response rates and prolong progression-free intervals. However, they also add complexity to the process of selecting treatment for individual patients at different stages of the disease process. Advanced and recurrent OC is rarely cured. Multiple lines of platinum combinations, and nonplatinum chemotherapeutics eventually fail to achieve clinical benefit, thus other active and tolerable systemic therapies are needed. Consequently, the US Food and Drug Administration has created a mechanism for "accelerated approval" of new medicines in situations of high unmet medical need.
Conclusion: We review the clinical implications of recent key clinical studies in these settings and outline the path forward for study design and approval of novel therapeutics to treat recurrent OC.
Keywords: BRCA1/2; Ovarian cancer; PARP; Platinum-refractory.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Monk discloses that he has received honoraria for speaker bureaus from Roche/Genentech, AstraZeneca, Janssen/Johnson & Johnson, Myriad, Clovis, and TESARO. Additionally, Dr. Monk has been a consultant for Roche/Genentech, Merck, TESARO, AstraZeneca, Gradalis, Advaxis, Cerulean, Amgen, Vermillion, ImmunoGen, Pfizer, Bayer, NuCana, Insys, GlaxoSmithKline, Verastem, Mateon (formally OxiGENE), Clovis, Precision Oncology, Perthera, Biodesix, and ImmunoGen. Dr. Herzog discloses that he has been a consultant for Roche, Johnson &Johnson, AstraZeneca, TESARO, Clovis, and Caris within past 1 year.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a southwest oncology group and gynecologic oncology group trial. J Clin Oncol. 2003;21(13):2460–2465. doi: 10.1200/JCO.2003.07.013. - DOI - PubMed
-
- Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a southwest oncology group and gynecologic oncology group phase 3 trial. Gynecol Oncol. 2009;114(2):195–198. doi: 10.1016/j.ygyno.2009.04.012. - DOI - PMC - PubMed
-
- Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al. Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. J Clin Oncol. 2009;27(28):4642–4648. doi: 10.1200/JCO.2009.21.9691. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

