Nasal high flow treatment in preterm infants
- PMID: 28904810
- PMCID: PMC5586012
- DOI: 10.1186/s40748-017-0056-y
Nasal high flow treatment in preterm infants
Abstract
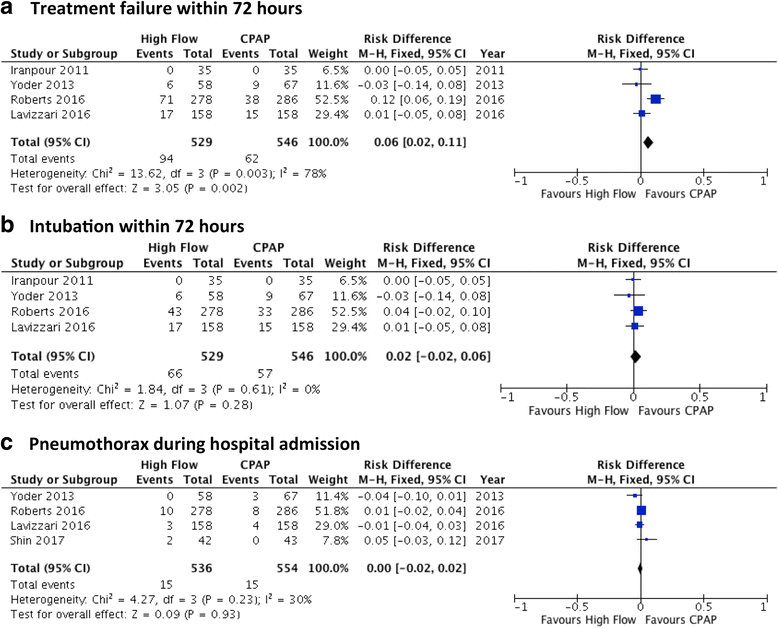
Nasal High Flow (HF) is a mode of 'non-invasive' respiratory support for preterm infants, with several potential modes of action, including generation of distending airway pressure, washout of the nasopharyngeal dead space, reduction of work of breathing, and heating and humidification of inspired gas. HF has several potential advantages over continuous positive airway pressure (CPAP), the most commonly applied form of non-invasive support, such as reduced nasal trauma, ease of use, and infant comfort, which has led to its rapid adoption into neonatal care. In recent years, HF has become a well-established and commonly applied treatment in neonatal care. Recent trials comparing HF and CPAP as primary support have had differing results. Meta-analyses suggest that primary HF results in an increased risk of treatment failure, but that 'rescue' CPAP use in those infants with HF failure results in no greater risk of mechanical ventilation. Even in studies with higher rates of HF failure, the majority of infants were successfully treated with HF, and rates of important neonatal morbidities did not differ between treatment groups. Importantly, these studies have included only infants born at ≥28 weeks' gestational age (GA). The decision whether to apply primary HF will depend on the value placed on its advantages over CPAP by clinicians, the approach to surfactant treatment, and the severity of respiratory disease in the relevant population of preterm infants. Post-extubation HF use results in similar rates of treatment failure, mechanical ventilation, and adverse events compared to CPAP. Post-extubation HF appears most suited to infants ≥28 weeks; there are few published data for infants below this gestation, and available evidence suggests that these infants are at high risk of HF failure, although rates of intubation and other morbidities are similar to those seen with CPAP. There is no evidence that using HF to 'wean' off CPAP allows for respiratory support to be ceased more quickly, but given its advantages it would appear to be a suitable alternative in infants who require ongoing non-invasive support. Safety data from randomised trials are reassuring, although more evidence in extremely preterm infants (<28 weeks' GA) is required.
Keywords: Continuous positive airway pressure; Infant, premature; Nasal high flow; Non-invasive ventilation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patient image included with this article is used with parental consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Protocol for a randomised controlled trial comparing two CPAP levels to prevent extubation failure in extremely preterm infants.BMJ Open. 2021 Jun 23;11(6):e045897. doi: 10.1136/bmjopen-2020-045897. BMJ Open. 2021. PMID: 34162644 Free PMC article.
-
High-flow nasal cannula: Mechanisms, evidence and recommendations.Semin Fetal Neonatal Med. 2016 Jun;21(3):139-45. doi: 10.1016/j.siny.2016.01.002. Epub 2016 Feb 9. Semin Fetal Neonatal Med. 2016. PMID: 26869581 Review.
-
A multicentre, randomised controlled, non-inferiority trial, comparing high flow therapy with nasal continuous positive airway pressure as primary support for preterm infants with respiratory distress (the HIPSTER trial): study protocol.BMJ Open. 2015 Jun 24;5(6):e008483. doi: 10.1136/bmjopen-2015-008483. BMJ Open. 2015. PMID: 26109120 Free PMC article. Clinical Trial.
-
High-flow support in very preterm infants in Australia and New Zealand.Arch Dis Child Fetal Neonatal Ed. 2016 Sep;101(5):F401-3. doi: 10.1136/archdischild-2015-309328. Epub 2015 Dec 17. Arch Dis Child Fetal Neonatal Ed. 2016. PMID: 26678879
-
Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants-A systematic review and meta-analysis.Pediatr Pulmonol. 2018 Jul;53(7):987-992. doi: 10.1002/ppul.24014. Epub 2018 Apr 23. Pediatr Pulmonol. 2018. PMID: 29687659
Cited by
-
Current insights in non-invasive ventilation for the treatment of neonatal respiratory disease.Ital J Pediatr. 2019 Aug 19;45(1):105. doi: 10.1186/s13052-019-0707-x. Ital J Pediatr. 2019. PMID: 31426828 Free PMC article. Review.
-
Non-Invasive Ventilation in Neonatology.Dtsch Arztebl Int. 2019 Mar 8;116(11):177-183. doi: 10.3238/arztebl.2019.0177. Dtsch Arztebl Int. 2019. PMID: 31014448 Free PMC article. Review.
-
Noninvasive Positive Airway Pressure Management for Post-extubation Support in Preterm Infants: Observational Cohort Study with Overlap Weighting Analysis.Ann Clin Epidemiol. 2023 Nov 10;6(1):17-23. doi: 10.37737/ace.24004. eCollection 2024. Ann Clin Epidemiol. 2023. PMID: 38605917 Free PMC article.
-
Strategies for the prevention of bronchopulmonary dysplasia.Front Pediatr. 2024 Jul 24;12:1439265. doi: 10.3389/fped.2024.1439265. eCollection 2024. Front Pediatr. 2024. PMID: 39114855 Free PMC article. Review.
-
Proficiency of Laryngeal Mask Airway Insertion Skill in NRP Certified Providers.Am J Perinatol. 2022 Jul;39(9):1008-1014. doi: 10.1055/s-0040-1721379. Epub 2020 Nov 29. Am J Perinatol. 2022. PMID: 33249550 Free PMC article.
References
-
- Davis PG, Henderson-Smart DJ. Nasal Continuous Positive Airways Pressure Immediately After Extubation For Preventing Morbidity In Preterm Infants. Cochrane Database Syst Rev 2003, Issue 2. Art. No.:Cd000143. doi:10.1002/14651858.CD000143. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous