Distribution of O-Acetylated Sialic Acids among Target Host Tissues for Influenza Virus
- PMID: 28904995
- PMCID: PMC5588038
- DOI: 10.1128/mSphere.00379-16
Distribution of O-Acetylated Sialic Acids among Target Host Tissues for Influenza Virus
Abstract
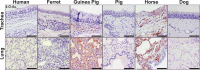
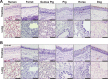
Sialic acids (Sias) are important glycans displayed on the cells and tissues of many different animals and are frequent targets for binding and modification by pathogens, including influenza viruses. Influenza virus hemagglutinins bind Sias during the infection of their normal hosts, while the encoded neuraminidases and/or esterases remove or modify the Sia to allow virion release or to prevent rebinding. Sias naturally occur in a variety of modified forms, and modified Sias can alter influenza virus host tropisms through their altered interactions with the viral glycoproteins. However, the distribution of modified Sia forms and their effects on pathogen-host interactions are still poorly understood. Here we used probes developed from viral Sia-binding proteins to detect O-acetylated (4-O-acetyl, 9-O-acetyl, and 7,9-O-acetyl) Sias displayed on the tissues of some natural or experimental hosts for influenza viruses. These modified Sias showed highly variable displays between the hosts and tissues examined. The 9-O-acetyl (and 7,9-) modified Sia forms were found on cells and tissues of many hosts, including mice, humans, ferrets, guinea pigs, pigs, horses, dogs, as well as in those of ducks and embryonated chicken egg tissues and membranes, although in variable amounts. The 4-O-acetyl Sias were found in the respiratory tissues of fewer animals, being primarily displayed in the horse and guinea pig, but were not detected in humans or pigs. The results suggest that these Sia variants may influence virus tropisms by altering and selecting their cell interactions. IMPORTANCE Sialic acids (Sias) are key glycans that control or modulate many normal cell and tissue functions while also interacting with a variety of pathogens, including many different viruses. Sias are naturally displayed in a variety of different forms, with modifications at several positions that can alter their functional interactions with pathogens. In addition, Sias are often modified or removed by enzymes such as host or pathogen esterases or sialidases (neuraminidases), and Sia modifications can alter those enzymatic activities to impact pathogen infections. Sia chemical diversity in different hosts and tissues likely alters the pathogen-host interactions and influences the outcome of infection. Here we explored the display of 4-O-acetyl, 9-O-acetyl, and 7,9-O-acetyl modified Sia forms in some target tissues for influenza virus infection in mice, humans, birds, guinea pigs, ferrets, swine, horses, and dogs, which encompass many natural and laboratory hosts of those viruses.
Keywords: host range; influenza; receptor-ligand interaction; respiratory viruses; sialic acid; virus-host interactions.
Figures







Similar articles
-
Modified Sialic Acids on Mucus and Erythrocytes Inhibit Influenza A Virus Hemagglutinin and Neuraminidase Functions.J Virol. 2020 Apr 16;94(9):e01567-19. doi: 10.1128/JVI.01567-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32051275 Free PMC article.
-
Expression of 9-O- and 7,9-O-Acetyl Modified Sialic Acid in Cells and Their Effects on Influenza Viruses.mBio. 2019 Dec 3;10(6):e02490-19. doi: 10.1128/mBio.02490-19. mBio. 2019. PMID: 31796537 Free PMC article.
-
Single Particle Analysis of H3N2 Influenza Entry Differentiates the Impact of the Sialic Acids (Neu5Ac and Neu5Gc) on Virus Binding and Membrane Fusion.J Virol. 2023 Mar 30;97(3):e0146322. doi: 10.1128/jvi.01463-22. Epub 2023 Feb 13. J Virol. 2023. PMID: 36779754 Free PMC article.
-
Effects of Sialic Acid Modifications on Virus Binding and Infection.Trends Microbiol. 2016 Dec;24(12):991-1001. doi: 10.1016/j.tim.2016.07.005. Epub 2016 Aug 1. Trends Microbiol. 2016. PMID: 27491885 Free PMC article. Review.
-
SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction.Int J Mol Sci. 2020 Jun 26;21(12):4549. doi: 10.3390/ijms21124549. Int J Mol Sci. 2020. PMID: 32604730 Free PMC article. Review.
Cited by
-
Mucins: the frontline defence of the lung.Biochem Soc Trans. 2018 Oct 19;46(5):1099-1106. doi: 10.1042/BST20170402. Epub 2018 Aug 28. Biochem Soc Trans. 2018. PMID: 30154090 Free PMC article. Review.
-
The role of 9-O-acetylated glycan receptor moieties in the typhoid toxin binding and intoxication.PLoS Pathog. 2020 Feb 21;16(2):e1008336. doi: 10.1371/journal.ppat.1008336. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32084237 Free PMC article.
-
Molecular Basis for Pathogenicity of Human Coronaviruses.Infect Drug Resist. 2020 Jul 17;13:2385-2405. doi: 10.2147/IDR.S255156. eCollection 2020. Infect Drug Resist. 2020. PMID: 32765013 Free PMC article. Review.
-
Influenza binds phosphorylated glycans from human lung.Sci Adv. 2019 Feb 13;5(2):eaav2554. doi: 10.1126/sciadv.aav2554. eCollection 2019 Feb. Sci Adv. 2019. PMID: 30788437 Free PMC article.
-
Modified Sialic Acids on Mucus and Erythrocytes Inhibit Influenza A Virus Hemagglutinin and Neuraminidase Functions.J Virol. 2020 Apr 16;94(9):e01567-19. doi: 10.1128/JVI.01567-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32051275 Free PMC article.
References
-
- Varki A, Schauer R. 2009. Sialic acids, p 199–218. In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
-
- Cummings RD, Turco S. 2009. Parasitic infections, p 553–566. In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
-
- Nizet V, Esko JD. 2009. Bacterial and viral infections, p 537–552. In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (ed), Essentials of glycobiology, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
