Lessons learned from protein aggregation: toward technological and biomedical applications
- PMID: 28905328
- PMCID: PMC5662053
- DOI: 10.1007/s12551-017-0317-z
Lessons learned from protein aggregation: toward technological and biomedical applications
Abstract
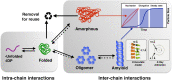
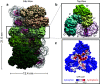
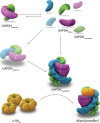
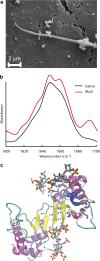
The close relationship between protein aggregation and neurodegenerative diseases has been the driving force behind the renewed interest in a field where biophysics, neurobiology and nanotechnology converge in the study of the aggregate state. On one hand, knowledge of the molecular principles that govern the processes of protein aggregation has a direct impact on the design of new drugs for high-incidence pathologies that currently can only be treated palliatively. On the other hand, exploiting the benefits of protein aggregation in the design of new nanomaterials could have a strong impact on biotechnology. Here we review the contributions of our research group on novel neuroprotective strategies developed using a purely biophysical approach. First, we examine how doxycycline, a well-known and innocuous antibiotic, can reshape α-synuclein oligomers into non-toxic high-molecular-weight species with decreased ability to destabilize biological membranes, affect cell viability and form additional toxic species. This mechanism can be exploited to diminish the toxicity of α-synuclein oligomers in Parkinson's disease. Second, we discuss a novel function in proteostasis for extracellular glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in combination with a specific glycosaminoglycan (GAG) present in the extracellular matrix. GAPDH, by changing its quaternary structure from a tetramer to protofibrillar assembly, can kidnap toxic species of α-synuclein, and thereby interfere with the spreading of the disease. Finally, we review a brighter side of protein aggregation, that of exploiting the physicochemical advantages of amyloid aggregates as nanomaterials. For this, we designed a new generation of insoluble biocatalysts based on the binding of photo-immobilized enzymes onto hybrid protein:GAG amyloid nanofibrils. These new nanomaterials can be easily functionalized by attaching different enzymes through dityrosine covalent bonds.
Keywords: Alzheimer’s disease; Amyloid; Amyloid functionalization; Cross-beta structure; Glycosaminoglycan; Parkinson’s disease; Protein aggregation.
Conflict of interest statement
César L Avila declares that he has no conflicts of interest. Silvina Chaves declares that she has no conflicts of interest. Sergio B Socias declares that he has no conflicts of interest. Esteban Vera-Pingitore declares that he has no conflicts of interest. Florencia González-Lizárraga declares that she has no conflicts of interest. Cecilia Vera declares that she has no conflicts of interest. Diego Ploper declares that he has no conflicts of interest. Rosana Chehín declares that she has no conflicts of interest.
Figures
References
-
- An B, Wang X, Cui M, Gui X, Mao X, Liu Y, Li K et al (2017) Diverse supramolecular nanofiber networks assembled by functional low-complexity domains. ACS Nano. 10.1021/acsnano.7b02298 - PubMed
-
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, et al. Phosphorylation of ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

