Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial
- PMID: 28905478
- PMCID: PMC5698740
- DOI: 10.1111/dom.13114
Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial
Abstract
Aims: To investigate the efficacy and safety of alirocumab in participants with type 2 (T2D) or type 1 diabetes (T1D) treated with insulin who have elevated LDL cholesterol levels despite maximally tolerated statin therapy.
Methods: Participants at high cardiovascular risk with T2D (n = 441) or T1D (n = 76) and LDL cholesterol levels ≥1.8 mmol/L (≥70 mg/dL) were randomized 2:1 to alirocumab:placebo administered subcutaneously every 2 weeks, for 24 weeks' double-blind treatment. Alirocumab-treated participants received 75 mg every 2 weeks, with blinded dose increase to 150 mg every 2 weeks at week 12 if week 8 LDL cholesterol levels were ≥1.8 mmol/L. Primary endpoints were percentage change in calculated LDL cholesterol from baseline to week 24, and safety assessments.
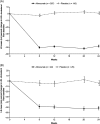
Results: Alirocumab reduced LDL cholesterol from baseline to week 24 by a mean ± standard error of 49.0% ± 2.7% and 47.8% ± 6.5% vs placebo (both P < .0001) in participants with T2D and T1D, respectively. Significant reductions were observed in non-HDL cholesterol (P < .0001), apolipoprotein B (P < .0001) and lipoprotein (a) (P ≤ .0039). At week 24, 76.4% and 70.2% of the alirocumab group achieved LDL cholesterol <1.8 mmol/L in the T2D and T1D populations (P < .0001), respectively. Glycated haemoglobin and fasting plasma glucose levels remained stable for the study duration. Treatment-emergent adverse events were observed in 64.5% of alirocumab- vs 64.1% of placebo-treated individuals (overall population).
Conclusions: Alirocumab produced significant LDL cholesterol reductions in participants with insulin-treated diabetes regardless of diabetes type, and was generally well tolerated. Concomitant administration of alirocumab and insulin did not raise any safety concerns (NCT02585778).
Keywords: cardiovascular disease; clinical trial; lipid-lowering therapy; type 1 diabetes; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
L. A. L. has received personal fees from Aegerion, grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Merck, Regeneron Pharmaceuticals, Inc. and Sanofi, and grants from Kowa and the Medicines Company, outside of this submitted work. B. C. has received research funding and personal fees from Sanofi and Regeneron Pharmaceuticals, Inc. during the conduct of the study, research funding from Pfizer, and honoraria from Amgen, AstraZeneca, Pierre Fabre, Janssen, Eli Lilly and Company, MSD (Merck & Co.), Novo Nordisk, Sanofi, and Takeda outside of this submitted work. D. M.‐W. has received speakers' bureau and consultant/advisory board fees from Amgen, AstraZeneca, Boehringer Ingelheim, MSD (Merck & Co.), Novartis, Novo Nordisk and Sanofi. H. M. C. has received grants, personal fees and non‐financial support from Sanofi and Regeneron Pharmaceuticals, Inc. during the conduct of this study, grants, personal fees and non‐financial support from Eli Lilly and Company, and grants from Roche Pharmaceuticals, Pfizer, Boehringer Ingelheim, AstraZeneca and Bayer, outside of this submitted work. S. D. P. has received research funding from Novartis Pharmaceuticals Co., MSD (Merck & Co.) and Novo Nordisk, and has been a consultant for or received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi‐Aventis and Takeda Pharmaceuticals. F. J. T. has received speakers' bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk and Sanofi‐Aventis. K. K. R. has received personal fees (data safety monitoring board) from AbbVie Inc., consultant fees/honoraria from Aegerion, Algorithm, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Eli Lilly and Company, Ionis Pharmaceuticals, Kowa, the Medicines Company, MSD (Merck & Co.), Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Reservlogix, Sanofi and Takeda, and research grants from Kowa, Pfizer and Regeneron Pharmaceuticals, Inc. M. B.‐B. and C. D. are employees of and shareholders in Sanofi. J. M. is an employee of IviData Stats, contracted to Sanofi. R. S. is an employee of and shareholder in Regeneron Pharmaceuticals, Inc. R. R. H. has received research funding from Eli Lilly and Company, Fuji Chemicals, Hitachi, Lexicon, Novo Nordisk, Pfizer and Viacyte, and has been a consultant and advisory panel member for Alere Inc., AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Elcelyx, Intarcia, Ionis, Janssen/Johnson & Johnson and Sanofi‐Aventis.
The authors were fully responsible for all content and editorial decisions, were involved in all stages of manuscript development, and have approved the final version.
Figures
Comment in
-
Lowering low-density lipoprotein cholesterol by PCSK9 inhibition in patients with diabetes on insulin therapy: is it efficacious and safe?Ann Transl Med. 2018 Feb;6(3):60. doi: 10.21037/atm.2018.01.02. Ann Transl Med. 2018. PMID: 29610751 Free PMC article. No abstract available.
References
-
- de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130:1110–1130. - PubMed
-
- Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035–3087. - PubMed
-
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. - PubMed
-
- Feingold KR, Grunfeld C. Diabetes and Dyslipidemia. In: De Groot LJ, Chrousos G, Dungan K, et al., eds. Endotext: South Dartmouth, MA: MDText.com Inc; 2000.
-
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical