Evaluation of Traditional and Contemporary Methods for Detecting Syphacia obvelata and Aspiculuris tetraptera in Laboratory Mice
- PMID: 28905712
- PMCID: PMC5250492
Evaluation of Traditional and Contemporary Methods for Detecting Syphacia obvelata and Aspiculuris tetraptera in Laboratory Mice
Abstract
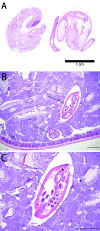
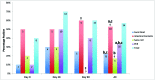
There is no consensus regarding the best practice for detecting murine pinworm infections. Initially, we evaluated 7 fecal concentration methods by using feces containing Aspiculuris tetraptera (AT) eggs (n = 20 samples per method). Sodium nitrate flotation, sodium nitrate centrifugation, Sheather sugar centrifugation, and zinc sulfate centrifugation detected eggs in 100% of samples; zinc sulfate flotation and water sedimentation detected eggs in 90%. All had better detection rates than Sheather sugar flotation (50%). To determine optimal detection methods, Swiss Webster mice were exposed to Syphacia obvelata (SO; n = 60) or AT (n = 60). We compared the following methods at days 0, 30, and 90, beginning 21 or 28 d after SO and AT exposure, respectively: fecal concentration (AT only), anal tape test (SO only), direct examination of intestinal contents (cecum and colon), Swiss roll histology (cecum and colon), and PCR analysis (pooled fur swab and feces). Detection rates for SO-exposed mice were: PCR analysis, 45%; Swiss roll histology, 30%; intestinal content exam, 27%; and tape test, 27%. The SO detection rate for PCR analysis was significantly greater than that for the tape test. Detection rates for AT-exposed mice were: intestinal content exam, 53%; PCR analysis, 33%; fecal flotation, 22%; and Swiss roll histology, 17%. The AT detection rate of PCR analysis combined with intestinal content examination was greater than for PCR analysis only and the AT detection rate of intestinal content examination was greater than for Swiss roll histology. Combining PCR analysis with intestinal content examination detected 100% of infected animals. No single test detected all positive animals. We recommend combining PCR analysis with intestinal content examination for optimal pinworm detection.
Figures

Similar articles
-
PCR Testing of IVC Filter Tops as a Method for Detecting Murine Pinworms and Fur Mites.J Am Assoc Lab Anim Sci. 2017 Nov 1;56(6):752-761. J Am Assoc Lab Anim Sci. 2017. PMID: 29256370 Free PMC article.
-
Guidance Regarding Sample Collection and Refinement of Fecal Flotation Exam for the Isolation of Aspiculuris tetraptera.J Am Assoc Lab Anim Sci. 2016;55(5):541-7. J Am Assoc Lab Anim Sci. 2016. PMID: 27657708 Free PMC article.
-
Molecular phylogeny of the pinworms of mice, rats and rabbits, and its use to develop molecular beacon assays for the detection of pinworms in mice.Lab Anim (NY). 2007 Oct;36(9):43-50. doi: 10.1038/laban1007-43. Lab Anim (NY). 2007. PMID: 17885663
-
Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification.Vet Parasitol. 2008 May 31;153(3-4):379-83. doi: 10.1016/j.vetpar.2008.02.001. Epub 2008 Feb 9. Vet Parasitol. 2008. PMID: 18374491
-
Pinworm infections in laboratory rodents: a review.Lab Anim. 1976 Jan;10(1):1-13. doi: 10.1258/002367776780948862. Lab Anim. 1976. PMID: 768631 Review.
Cited by
-
Evaluation of In-cage Filter Paper as a Replacement for Sentinel Mice in the Detection of Murine Pathogens.J Am Assoc Lab Anim Sci. 2021 Mar 1;60(2):160-167. doi: 10.30802/AALAS-JAALAS-20-000086. Epub 2021 Feb 24. J Am Assoc Lab Anim Sci. 2021. PMID: 33629939 Free PMC article.
-
PCR Testing of IVC Filter Tops as a Method for Detecting Murine Pinworms and Fur Mites.J Am Assoc Lab Anim Sci. 2017 Nov 1;56(6):752-761. J Am Assoc Lab Anim Sci. 2017. PMID: 29256370 Free PMC article.
-
PCR and RT-PCR in the Diagnosis of Laboratory Animal Infections and in Health Monitoring.J Am Assoc Lab Anim Sci. 2020 Sep 1;59(5):458-468. doi: 10.30802/AALAS-JAALAS-20-000008. Epub 2020 Jun 24. J Am Assoc Lab Anim Sci. 2020. PMID: 32580820 Free PMC article.
-
Comparing Mouse Health Monitoring Between Soiled-bedding Sentinel and Exhaust Air Dust Surveillance Programs.J Am Assoc Lab Anim Sci. 2020 Jan 1;59(1):58-66. doi: 10.30802/AALAS-JAALAS-19-000061. Epub 2019 Dec 20. J Am Assoc Lab Anim Sci. 2020. PMID: 31862019 Free PMC article.
-
Comparison of Diagnostic Methods and Sampling Sites for the Detection of Demodex musculi.J Am Assoc Lab Anim Sci. 2018 Mar 1;57(2):173-185. J Am Assoc Lab Anim Sci. 2018. PMID: 29555007 Free PMC article.
References
-
- Anya AO. 1966. Studies on the biology of some oxyurid nematodes. I. Factors in the development of eggs of Aspiculuris tetraptera Schulz. J Helminthol 40:253–260. - PubMed
-
- Anya AO. 1966. Studies on the biology of some oxyurid nematodes. II. The hatching of eggs and development of Aspiculuris tetraptera Schulz within the host. J Helminthol 40:261–268. - PubMed
-
- Baker DG. 2007. Parasites of rats and mice, p 303–397, Chapter 11. In: Baker DG. Flynn's parasites of laboratory animals. 2nd ed. Ames (IA): Blackwell Publishing.
-
- Behnke JM. 1974. The distribution of larval Aspiculuris tetraptera Schulz during a primary infection in Mus musculus, Rattus norvegicus, and Apodemus sylvaticus. Parasitology 69:391–402. - PubMed
-
- Behnke JM. 1975. Aspiculuris tetraptera in wild Mus musculus. The prevalence of infection in male and female mice. J Helminthol 49:85–90. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
