Structural behavior of competitive temperature and pH-responsive tethered polymer layers
- PMID: 28905971
- PMCID: PMC5712476
- DOI: 10.1039/c7sm01538k
Structural behavior of competitive temperature and pH-responsive tethered polymer layers
Abstract
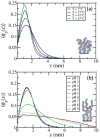
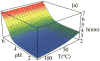
Herein, we develop a molecular theory to examine a class of pH and temperature-responsive tethered polymer layers. The response of pH depends on intramolecular charge repulsion of weakly acidic monomers and the response of temperature depends on hydrogen bonding between polymer monomers and water molecules akin to the behavior of water-soluble polymers such as PEG (poly-ethylene glycol) or NIPAAm (n-isopropylacrylamide). We investigate the changes in structural behavior that result for various end-tethered copolymers: pH/T responsive monomers alone, in alternating sequence with hydrophobic monomers, and as 50/50 diblocks with hydrophobic monomers. We find that the sequence and location of hydrophobic units play a critical role in the thermodynamic stability and structural behavior of these responsive polymer layers. Additionally, the polymers exhibit tunable collapse when varying the surface coverage, location and sequence of hydrophobic units as a function of temperature and pH. As far as we know, our results present the first molecularly detailed theory for end-tethered polymers that are both pH and temperature-responsive via hydrogen bonding. We propose that this work holds predictive power for the guided design of future biomaterials.
Figures





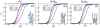

References
-
- Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. J Cell Physiol. 2005;203:465–470. - PubMed
-
- Qazi TH, Rai R, Boccaccini AR. Biomaterials. 2014;35:9068–9086. - PubMed
-
- Cohen Stuart MA, Huck WT, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nat Mater. 2010;9:101–113. - PubMed
-
- Kadokawa J, Saitou S, Shoda S. Carbohyd Polym. 2005;60:253–258.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

