Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes
- PMID: 28906039
- PMCID: PMC5766282
- DOI: 10.1002/glia.23210
Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes
Abstract
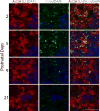
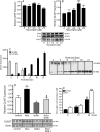
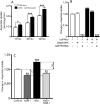
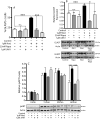
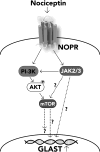
Our previous results showed that oligodendrocyte development is regulated by both nociceptin and its G-protein coupled receptor, the nociceptin/orphanin FQ receptor (NOR). The present in vitro and in vivo findings show that nociceptin plays a crucial conserved role regulating the levels of the glutamate/aspartate transporter GLAST/EAAT1 in both human and rodent brain astrocytes. This nociceptin-mediated response takes place during a critical developmental window that coincides with the early stages of astrocyte maturation. GLAST/EAAT1 upregulation by nociceptin is mediated by NOR and the downstream participation of a complex signaling cascade that involves the interaction of several kinase systems, including PI-3K/AKT, mTOR, and JAK. Because GLAST is the main glutamate transporter during brain maturation, these novel findings suggest that nociceptin plays a crucial role in regulating the function of early astrocytes and their capacity to support glutamate homeostasis in the developing brain.
Keywords: GLAST; astrocytes; brain development; glutamate transporters; nociceptin.
© 2017 Wiley Periodicals, Inc.
Figures







Similar articles
-
Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway.Exp Neurol. 2015 Nov;273:288-300. doi: 10.1016/j.expneurol.2015.09.009. Epub 2015 Sep 21. Exp Neurol. 2015. PMID: 26391563
-
Oncostatin M promotes excitotoxicity by inhibiting glutamate uptake in astrocytes: implications in HIV-associated neurotoxicity.J Neuroinflammation. 2016 Jun 10;13(1):144. doi: 10.1186/s12974-016-0613-8. J Neuroinflammation. 2016. PMID: 27287400 Free PMC article.
-
Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway.J Neurosci. 2011 Dec 7;31(49):17864-71. doi: 10.1523/JNEUROSCI.3179-11.2011. J Neurosci. 2011. PMID: 22159101 Free PMC article.
-
Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system.Peptides. 2000 Jul;21(7):1023-9. doi: 10.1016/s0196-9781(00)00233-3. Peptides. 2000. PMID: 10998536 Review.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
Cited by
-
The role of gut-immune-brain signaling in substance use disorders.Int Rev Neurobiol. 2021;157:311-370. doi: 10.1016/bs.irn.2020.09.005. Epub 2020 Oct 24. Int Rev Neurobiol. 2021. PMID: 33648673 Free PMC article.
-
Cognitive Dysfunction After Analgesia and Sedation: Out of the Operating Room and Into the Pediatric Intensive Care Unit.Front Behav Neurosci. 2021 Aug 16;15:713668. doi: 10.3389/fnbeh.2021.713668. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34483858 Free PMC article. Review.
-
Nociceptin Receptor Is Overexpressed in Non-small Cell Lung Cancer and Predicts Poor Prognosis.Front Oncol. 2019 Apr 5;9:235. doi: 10.3389/fonc.2019.00235. eCollection 2019. Front Oncol. 2019. PMID: 31024840 Free PMC article.
-
Yin Yang 1 controls cerebellar astrocyte maturation.Glia. 2023 Oct;71(10):2437-2455. doi: 10.1002/glia.24434. Epub 2023 Jul 7. Glia. 2023. PMID: 37417428 Free PMC article.
-
Effects of novel beta-lactam, MC-100093, and ceftriaxone on astrocytic glutamate transporters and neuroinflammatory factors in nucleus accumbens of C57BL/6 mice exposed to escalated doses of morphine.Saudi Pharm J. 2024 Jul;32(7):102108. doi: 10.1016/j.jsps.2024.102108. Epub 2024 May 23. Saudi Pharm J. 2024. PMID: 38868175 Free PMC article.
References
-
- Aparicio LC, Candeletti S, Binaschi A, Mazzuferi M, Mantovani S, Di Benedetto M, Landuzzi D, Lopetuso G, Romualdi P, Simonato M. Kainate seizures increase nociceptin/orphanin FQ release in the rat hippocampus and thalamus: A microdialysis study. J Neurochem. 2004;91:30–37. - PubMed
-
- Aschner M. Neuron-astrocyte interactions: implications for cellular energetics and antioxidant levels. Neurotoxicology. 2000;21:1101–7. - PubMed
-
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. J Neurochem. 1997;69:2571–80. - PubMed
-
- Bayrakdar ET, Bojnik E, Armagan G, Kanit L, Benyhe S, Borsodi A, Yalcin A. Kainic acid-induced seizure activity alters the mRNA expression and G-protein activation of the opioid/nociceptin receptors in the rat brain cortex. Epilepsy Res. 2013;105:13–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

