The value of point-of-care CD4+ and laboratory viral load in tailoring antiretroviral therapy monitoring strategies to resource limitations
- PMID: 28906279
- PMCID: PMC5634708
- DOI: 10.1097/QAD.0000000000001586
The value of point-of-care CD4+ and laboratory viral load in tailoring antiretroviral therapy monitoring strategies to resource limitations
Abstract
Objective: To examine the clinical and economic value of point-of-care CD4 (POC-CD4) or viral load monitoring compared with current practices in Mozambique, a country representative of the diverse resource limitations encountered by HIV treatment programs in sub-Saharan Africa.
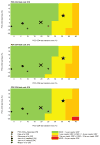
Design/methods: We use the Cost-Effectiveness of Preventing AIDS Complications-International model to examine the clinical impact, cost (2014 US$), and incremental cost-effectiveness ratio [$/year of life saved (YLS)] of ART monitoring strategies in Mozambique. We compare: monitoring for clinical disease progression [clinical ART monitoring strategy (CLIN)] vs. annual POC-CD4 in rural settings without laboratory services and biannual laboratory CD4 (LAB-CD4), biannual POC-CD4, and annual viral load in urban settings with laboratory services. We examine the impact of a range of values in sensitivity analyses, using Mozambique's 2014 per capita gross domestic product ($620) as a benchmark cost-effectiveness threshold.
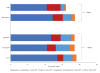
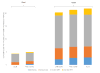
Results: In rural settings, annual POC-CD4 compared to CLIN improves life expectancy by 2.8 years, reduces time on failed ART by 0.6 years, and yields an incremental cost-effectiveness ratio of $480/YLS. In urban settings, biannual POC-CD4 is more expensive and less effective than viral load. Compared to biannual LAB-CD4, viral load improves life expectancy by 0.6 years, reduces time on failed ART by 1.0 year, and is cost-effective ($440/YLS).
Conclusion: In rural settings, annual POC-CD4 improves clinical outcomes and is cost-effective compared to CLIN. In urban settings, viral load has the greatest clinical benefit and is cost-effective compared to biannual POC-CD4 or LAB-CD4. Tailoring ART monitoring strategies to specific settings with different available resources can improve clinical outcomes while remaining economically efficient.
Conflict of interest statement
Figures
Similar articles
-
The clinical and economic impact of point-of-care CD4 testing in mozambique and other resource-limited settings: a cost-effectiveness analysis.PLoS Med. 2014 Sep 16;11(9):e1001725. doi: 10.1371/journal.pmed.1001725. eCollection 2014 Sep. PLoS Med. 2014. PMID: 25225800 Free PMC article.
-
CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study.BMJ. 2011 Nov 9;343:d6884. doi: 10.1136/bmj.d6884. BMJ. 2011. PMID: 22074713 Free PMC article. Clinical Trial.
-
Laboratory Monitoring of Antiretroviral Therapy for HIV Infection: Cost-Effectiveness and Budget Impact of Current and Novel Strategies.Clin Infect Dis. 2016 Jun 1;62(11):1454-1462. doi: 10.1093/cid/ciw117. Epub 2016 Mar 1. Clin Infect Dis. 2016. PMID: 26936666 Free PMC article.
-
Economic evaluation of ART in resource-limited countries.Curr Opin HIV AIDS. 2010 May;5(3):225-31. doi: 10.1097/COH.0b013e3283384a9d. Curr Opin HIV AIDS. 2010. PMID: 20539078 Free PMC article. Review.
-
Challenges in implementing HIV laboratory monitoring in resource-constrained settings: how to do more with less.Future Microbiol. 2011 Nov;6(11):1251-60. doi: 10.2217/fmb.11.121. Future Microbiol. 2011. PMID: 22082287 Review.
Cited by
-
Health Economic Evidence of Point-of-Care Testing: A Systematic Review.Pharmacoecon Open. 2021 Jun;5(2):157-173. doi: 10.1007/s41669-020-00248-1. Epub 2021 Jan 6. Pharmacoecon Open. 2021. PMID: 33405188 Free PMC article.
-
Advanced in immunological monitoring of HIV infection: profile of immune cells and cytokines in people living with HIV-1 in Benin.BMC Immunol. 2024 Apr 20;25(1):22. doi: 10.1186/s12865-024-00615-1. BMC Immunol. 2024. PMID: 38643073 Free PMC article.
-
Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study.Lancet HIV. 2019 Mar;6(3):e182-e190. doi: 10.1016/S2352-3018(18)30328-X. Epub 2019 Feb 5. Lancet HIV. 2019. PMID: 30737187 Free PMC article.
-
Factors Associated With Viral Suppression and Drug Resistance in Children and Adolescents Living With HIV in Care and Treatment Programs in Southern Tanzania.J Pediatric Infect Dis Soc. 2023 Jun 30;12(6):353-363. doi: 10.1093/jpids/piad040. J Pediatric Infect Dis Soc. 2023. PMID: 37279560 Free PMC article.
-
Clinic-based SAMBA-II vs centralized laboratory viral load assays among HIV-1 infected children, adolescents and young adults in rural Zimbabwe: A randomized controlled trial.PLoS One. 2023 Feb 14;18(2):e0281279. doi: 10.1371/journal.pone.0281279. eCollection 2023. PLoS One. 2023. PMID: 36787296 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016 [6/15/2017]. Available from: http://www.who.int/hiv/pub/guidelines/keypopulations-2016/en/ - PubMed
-
- Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Mbougua JB, Boyer S, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis. 2011 Nov;11(11):825–33. Epub 2011/08/13. - PubMed
-
- Alere. Studies & Implementation. 2017 [6/14/2017]. Available from: http://www.alerehiv.com/ww/home/studies-and-implementation.html.
-
- UNAIDS. Resposta global à SIDA relatório do progresso, 2016 Moçambique United Nations. 2016 [cited 6/15/2017]. Available from: http://www.unaids.org/sites/default/files/country/documents/MOZ_narrativ....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

