Polyethylene Glycol 3350 With Electrolytes Versus Polyethylene Glycol 4000 for Constipation: A Randomized, Controlled Trial
- PMID: 28906317
- PMCID: PMC5753830
- DOI: 10.1097/MPG.0000000000001726
Polyethylene Glycol 3350 With Electrolytes Versus Polyethylene Glycol 4000 for Constipation: A Randomized, Controlled Trial
Abstract
Objective: The long-term efficacy and safety of polyethylene glycol (PEG) in constipated children are unknown, and a head-to-head comparison of the different PEG formulations is lacking. We aimed to investigate noninferiority of PEG3350 with electrolytes (PEG3350 + E) compared to PEG4000 without electrolytes (PEG4000).
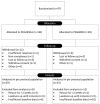
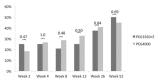
Methods: In this double-blind trial, children aged 0.5 to 16 years with constipation, defined as a defecation frequency of <3 times per week, were randomized to receive either PEG3350 + E or PEG4000. Primary outcomes were change in total sum score (TSS) at week 52 compared to baseline, and dose range determination. TSS was the sum of the severity of 5 constipation symptoms rated on a 4-point scale (0-3). Noninferiority margin was a difference in TSS of ≤1.5 based on a 95%-confidence interval [CI]. Treatment success was defined as a defecation frequency of ≥3 per week with <1 episode of fecal incontinence.
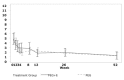
Results: Ninety-seven subjects were included, of whom 82 completed the study. Mean reduction in TSS was -3.81 (95% CI: -4.96 to -2.65) and -3.74 (95%CI: -5.08 to -2.40), for PEG3350 + E and PEG4000, respectively. Noninferiority criteria were not met (maximum difference between groups: -1.81 to 1.68). Daily sachet use was: 0 to 2 years: 0.4 to 2.3 and 0.9 to 2.1; 2 to 4 years: 0.1 to 3.5 and 1.2 to 3.2; 4 to 8 years: 1.1 to 2.8 and 0.7 to 3.8; 8 to 16 years 0.6 to 3.7 and 1.0 to 3.7, in PEG3350 + E and PEG4000, respectively. Treatment success after 52 weeks was achieved in 50% and 45% of children, respectively (P = 0.69). Rates of adverse events were similar between groups, and no drug-related serious adverse events occurred.
Conclusions: Noninferiority regarding long-term constipation-related symptoms of PEG3350 + E compared to PEG4000 was not demonstrated. However, analysis of secondary outcomes suggests similar efficacy and safety of these agents.
Trial registration: ClinicalTrials.gov NCT01810653.
Conflict of interest statement
M.V. is employed by Bayer. The other authors report no conflict of interest.
Figures
References
-
- Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011; 25:3–18. - PubMed
-
- Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014; 58:258–274. - PubMed
-
- Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev 2010; CD007570. - PubMed
-
- Brady CE, DiPalma JA, Morawski SG, et al. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 1986; 90:1914–1918. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

