Visible-Light-Promoted C-S Cross-Coupling via Intermolecular Charge Transfer
- PMID: 28910097
- PMCID: PMC5920654
- DOI: 10.1021/jacs.7b07390
Visible-Light-Promoted C-S Cross-Coupling via Intermolecular Charge Transfer
Abstract
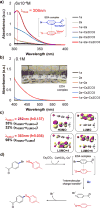
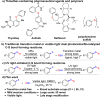
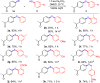
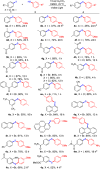
Disclosed is a mild, scalable, visible-light-promoted cross-coupling reaction between thiols and aryl halides for the construction of C-S bonds in the absence of both transition metal and photoredox catalysts. The scope of aryl halides and thiol partners includes over 60 examples and therefore provides an entry point into various aryl thioether building blocks of pharmaceutical interest. Furthermore, to demonstrate its utility, this C-S coupling protocol was applied in drug synthesis and late-stage modifications of active pharmaceutical ingredients. UV-vis spectroscopy and time-dependent density functional theory calculations suggest that visible-light-promoted intermolecular charge transfer within the thiolate-aryl halide electron donor-acceptor complex permits the reactivity in the absence of catalyst.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
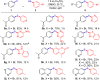
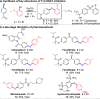
References
-
- Feng M, Tang B, Liang S, Jiang X. Curr Top Med Chem. 2016;16:1200. - PMC - PubMed
- Patani GA, LaVoie EJ. Chem Rev. 1996;96:3147. - PubMed
- Ilardi EA, Vitaku E, Njardarson JT. J Med Chem. 2014;57:2832. - PubMed
- Boyd DA. Angew Chem, Int Ed. 2016;55:15486. - PubMed
- Rahate AS, Nemade KR, Waghuley SA. Rev Chem Eng. 2013;29:471.
-
- Kwong FY, Buchwald SL. Org Lett. 2002;4:3517. - PubMed
- Murata M, Buchwald SL. Tetrahedron. 2004;60:7397.
- Fernańdez-Rodríguez MA, Shen Q, Hartwig JF. J Am Chem Soc. 2006;128:2180. - PubMed
- Alvaro E, Hartwig JF. J Am Chem Soc. 2009;131:7858. - PMC - PubMed
- Sayah M, Organ MG. Chem – Eur J. 2011;17:11719. - PubMed
- Gogoi P, Hazarika S, Sarma MJ, Sarma K, Barman P. Tetrahedron. 2014;70:7484.
-
- Wang X, Cuny GD, Noël T. Angew Chem, Int Ed. 2013;52:7860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

