Fast and Simple Protocols for Mass Spectrometry-Based Proteomics of Small Fresh Frozen Uterine Tissue Sections
- PMID: 28910098
- PMCID: PMC5647562
- DOI: 10.1021/acs.analchem.7b01937
Fast and Simple Protocols for Mass Spectrometry-Based Proteomics of Small Fresh Frozen Uterine Tissue Sections
Abstract
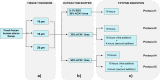
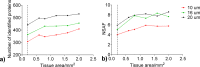
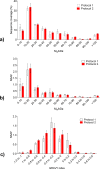
Human tissues are an important link between organ-specific spatial molecular information, patient pathology, and patient treatment options. However, patient tissues are uniquely obtained by time and location, and limited in their availability and size. Currently, little knowledge exists about appropriate and simplified protocols for routine MS-based analysis of the various types and sizes of tissues. Following standard procedures used in pathology, we selected small fresh frozen uterine tissue samples to investigate how the tissue preparation protocol affected the subsequent proteomics analysis. First, we observed that protein extraction with 0.1% SDS followed by extraction with a 30% ACN/urea resulted in a decrease in the number of identified proteins, when compared to extraction with 30% ACN/urea only. The decrease in the number of proteins was approximately 55% and 20%, for 10 and 16 μm thick tissue, respectively. Interestingly, the relative abundance of the proteins shared between the two methods was higher when SDS/ACN/urea was used, compared to the 30% ACN/urea extraction, indicating the role of SDS to be beneficial for protein solubility. Second, the influence of tissue thickness was investigated by comparing the results obtained for 10, 16, and 20 μm thick (1 mm2) tissue using urea/30% ACN. We observed an increase in the number of identified proteins and corresponding quantity with an increase in the tissue thickness. Finally, by analyzing very small amounts of tissues (∼0.2 mm2) of 10, 16, and 20 μm thickness, we observed that the increase in tissue thickness resulted in a higher number of protein identifications and corresponding quantitative values.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

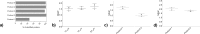
Similar articles
-
Protocol for the Analysis of Laser Capture Microdissected Fresh-Frozen Tissue Homogenates by Silver-Stained 1D SDS-PAGE.Methods Mol Biol. 2018;1723:95-110. doi: 10.1007/978-1-4939-7558-7_4. Methods Mol Biol. 2018. PMID: 29344855
-
Enrichment of low molecular weight serum proteins using acetonitrile precipitation for mass spectrometry based proteomic analysis.Rapid Commun Mass Spectrom. 2008 Oct;22(20):3255-60. doi: 10.1002/rcm.3729. Rapid Commun Mass Spectrom. 2008. PMID: 18803344
-
Extraction efficiency and implications for absolute quantitation of propranolol in mouse brain, liver and kidney tissue sections using droplet-based liquid microjunction surface sampling high-performance liquid chromatography/electrospray ionization tandem mass spectrometry.Rapid Commun Mass Spectrom. 2016 Jul 30;30(14):1705-1712. doi: 10.1002/rcm.7607. Rapid Commun Mass Spectrom. 2016. PMID: 28328034
-
The benefits (and misfortunes) of SDS in top-down proteomics.J Proteomics. 2018 Mar 20;175:75-86. doi: 10.1016/j.jprot.2017.03.002. Epub 2017 Mar 8. J Proteomics. 2018. PMID: 28286130 Review.
-
Translational value of liquid chromatography coupled with tandem mass spectrometry-based quantitative proteomics for in vitro-in vivo extrapolation of drug metabolism and transport and considerations in selecting appropriate techniques.Expert Opin Drug Metab Toxicol. 2015;11(9):1357-69. doi: 10.1517/17425255.2015.1055245. Epub 2015 Jun 26. Expert Opin Drug Metab Toxicol. 2015. PMID: 26108733 Review.
Cited by
-
Hanging drop sample preparation improves sensitivity of spatial proteomics.Lab Chip. 2022 Jul 26;22(15):2869-2877. doi: 10.1039/d2lc00384h. Lab Chip. 2022. PMID: 35838077 Free PMC article.
-
Comprehensive spectral libraries for various rabbit eye tissue proteomes.Sci Data. 2022 Mar 29;9(1):111. doi: 10.1038/s41597-022-01241-5. Sci Data. 2022. PMID: 35351915 Free PMC article.
-
Translational and Posttranslational Dynamics in a Model Peptidergic System.Mol Cell Proteomics. 2023 May;22(5):100544. doi: 10.1016/j.mcpro.2023.100544. Epub 2023 Apr 6. Mol Cell Proteomics. 2023. PMID: 37030596 Free PMC article.
-
Development of a Sensitive, Scalable Method for Spatial, Cell-Type-Resolved Proteomics of the Human Brain.J Proteome Res. 2019 Apr 5;18(4):1787-1795. doi: 10.1021/acs.jproteome.8b00981. Epub 2019 Feb 25. J Proteome Res. 2019. PMID: 30768908 Free PMC article.
-
Evaluation of Fast and Sensitive Proteome Profiling of FF and FFPE Kidney Patient Tissues.Molecules. 2022 Feb 8;27(3):1137. doi: 10.3390/molecules27031137. Molecules. 2022. PMID: 35164409 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

