Stereoselective and Regioselective Synthesis of Heterocycles via Copper-Catalyzed Additions of Amine Derivatives and Alcohols to Alkenes
- PMID: 28910106
- PMCID: PMC5782808
- DOI: 10.1021/acs.joc.7b02072
Stereoselective and Regioselective Synthesis of Heterocycles via Copper-Catalyzed Additions of Amine Derivatives and Alcohols to Alkenes
Abstract
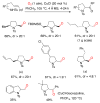
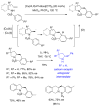
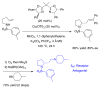
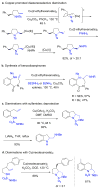
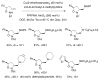
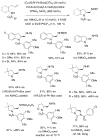
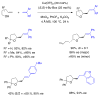
This Perspective describes the development of a family of copper(II)-catalyzed alkene difunctionalization reactions that enable stereoselective addition of amine derivatives and alcohols onto pendant unactivated alkenes to provide a range of valuable saturated nitrogen and oxygen heterocycles. 2-Vinylanilines and related substrates undergo alternative oxidative amination or allylic amination pathways, and these reactions will also be discussed. The involvement of both polar and radical steps in the reaction mechanisms have been implicated. Major product formation is a function of the lowest energy pathway, which in turn is a function of structural aspects of the various reaction components.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

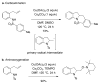

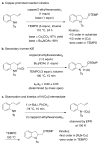


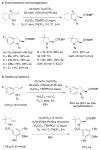
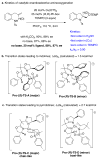



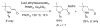



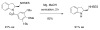
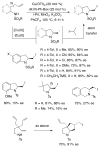

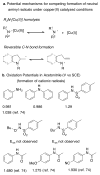

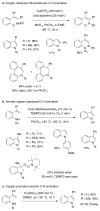
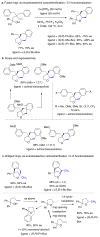


References
-
- Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257–10274. - PubMed
-
- de la Torre A, Cuyamendous C, Bultel-Ponce V, Durand T, Galano JM, Oger C. Tetrahedron. 2016;72:5003–5025.
-
- Ritchie TJ, Macdonald SJF. Drug Discovery Today. 2009;14:1011–1020. - PubMed
-
- Sanchez-Rosello M, Miro J, del Pozo C. Synthesis. 2017;49:2787–2802.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

