Acute and chronic effects of treatment with mesenchymal stromal cells on LPS-induced pulmonary inflammation, emphysema and atherosclerosis development
- PMID: 28910300
- PMCID: PMC5598950
- DOI: 10.1371/journal.pone.0183741
Acute and chronic effects of treatment with mesenchymal stromal cells on LPS-induced pulmonary inflammation, emphysema and atherosclerosis development
Abstract
Background: COPD is a pulmonary disorder often accompanied by cardiovascular disease (CVD), and current treatment of this comorbidity is suboptimal. Systemic inflammation in COPD triggered by smoke and microbial exposure is suggested to link COPD and CVD. Mesenchymal stromal cells (MSC) possess anti-inflammatory capacities and MSC treatment is considered an attractive treatment option for various chronic inflammatory diseases. Therefore, we investigated the immunomodulatory properties of MSC in an acute and chronic model of lipopolysaccharide (LPS)-induced inflammation, emphysema and atherosclerosis development in APOE*3-Leiden (E3L) mice.
Methods: Hyperlipidemic E3L mice were intranasally instilled with 10 μg LPS or vehicle twice in an acute 4-day study, or twice weekly during 20 weeks Western-type diet feeding in a chronic study. Mice received 0.5x106 MSC or vehicle intravenously twice after the first LPS instillation (acute study) or in week 14, 16, 18 and 20 (chronic study). Inflammatory parameters were measured in bronchoalveolar lavage (BAL) and lung tissue. Emphysema, pulmonary inflammation and atherosclerosis were assessed in the chronic study.
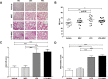
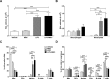
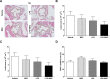
Results: In the acute study, intranasal LPS administration induced a marked systemic IL-6 response on day 3, which was inhibited after MSC treatment. Furthermore, MSC treatment reduced LPS-induced total cell count in BAL due to reduced neutrophil numbers. In the chronic study, LPS increased emphysema but did not aggravate atherosclerosis. Emphysema and atherosclerosis development were unaffected after MSC treatment.
Conclusion: These data show that MSC inhibit LPS-induced pulmonary and systemic inflammation in the acute study, whereas MSC treatment had no effect on inflammation, emphysema and atherosclerosis development in the chronic study.
Conflict of interest statement
Figures




References
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available from: http://www.goldcopd.org/. 2016. - PubMed
-
- Vivodtzev I, Tamisier R, Baguet JP, Borel JC, Levy P, Pepin JL. Arterial stiffness in COPD. Chest. 2014;145(4):861–75. doi: 1852921 [pii]; doi: 10.1378/chest.13-1809 - DOI - PubMed
-
- Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139(1):165–73. doi: 139/1/165 [pii]; doi: 10.1378/chest.10-1252 - DOI - PubMed
-
- Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116(2):307–11. doi: CIRCRESAHA.116.301313 [pii]; doi: 10.1161/CIRCRESAHA.116.301313 - DOI - PMC - PubMed
-
- Barua RS, Sharma M, Dileepan KN. Cigarette Smoke Amplifies Inflammatory Response and Atherosclerosis Progression Through Activation of the H1R-TLR2/4-COX2 Axis. Front Immunol. 2015;6:572 doi: 10.3389/fimmu.2015.00572 ; PubMed Central PMCID: PMCPMC4638143. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

