IκK-16 decreases miRNA-155 expression and attenuates the human monocyte inflammatory response
- PMID: 28910312
- PMCID: PMC5598939
- DOI: 10.1371/journal.pone.0183987
IκK-16 decreases miRNA-155 expression and attenuates the human monocyte inflammatory response
Abstract
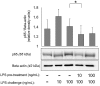
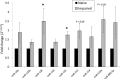
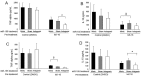
Excessive inflammatory responses in the surgical patient may result in cellular hypo-responsiveness, which is associated with an increased risk of secondary infection and death. microRNAs (miRNAs), such as miR-155, are powerful regulators of inflammatory signalling pathways including nuclear factor κB (NFκB). Our objective was to determine the effect of IκK-16, a selective blocker of inhibitor of kappa-B kinase (IκK), on miRNA expression and the monocyte inflammatory response. In a model of endotoxin tolerance using primary human monocytes, impaired monocytes had decreased p65 expression with suppressed TNF-α and IL-10 production (P < 0.05). miR-155 and miR-138 levels were significantly upregulated at 17 h in the impaired monocyte (P < 0.05). Notably, IκK-16 decreased miR-155 expression with a corresponding dose-dependent decrease in TNF-α and IL-10 production (P < 0.05), and impaired monocyte function was associated with increased miR-155 and miR-138 expression. In the context of IκK-16 inhibition, miR-155 mimics increased TNF-α production, while miR-155 antagomirs decreased both TNF-α and IL-10 production. These data demonstrate that IκK-16 treatment attenuates the monocyte inflammatory response, which may occur through a miR-155-mediated mechanism, and that IκK-16 is a promising approach to limit the magnitude of an excessive innate inflammatory response to LPS.
Conflict of interest statement
Figures





References
-
- Polinder S, Haagsma JA, Toet H, van Beeck EF. Epidemiological burden of minor, major and fatal trauma in a national injury pyramid. The British journal of surgery. 2012;99 Suppl 1:114–21. Epub 2012/03/28. doi: 10.1002/bjs.7708 . - DOI - PubMed
-
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354 ; PubMed Central PMCID: PMCPMC3244029. - DOI - PMC - PubMed
-
- Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, et al. Trauma deaths in a mature urban trauma system: is "trimodal" distribution a valid concept? J Am Coll Surg. 2005;201(3):343–8. doi: 10.1016/j.jamcollsurg.2005.05.003 . - DOI - PubMed
-
- Galbraith N, Walker S, Galandiuk S, Gardner S, Polk HC Jr. The Significance and Challenges of Monocyte Impairment: For the Ill Patient and the Surgeon. Surgical infections. 2016;17(3):303–12. Epub 2016/03/10. doi: 10.1089/sur.2015.245 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

