Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation
- PMID: 28910401
- PMCID: PMC5598993
- DOI: 10.1371/journal.pone.0184663
Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation
Abstract
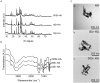
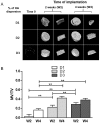
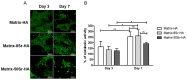
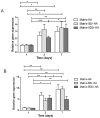
Previous studies performed using polysaccharide-based matrices supplemented with hydroxyapatite (HA) particles showed their ability to form in subcutaneous and intramuscular sites a mineralized and osteoid tissue. Our objectives are to optimize the HA content in the matrix and to test the combination of HA with strontium (Sr-HA) to increase the matrix bioactivity. First, non-doped Sr-HA powders were combined to the matrix at three different ratios and were implanted subcutaneously for 2 and 4 weeks. Interestingly, matrices showed radiolucent properties before implantation. Quantitative analysis of micro-CT data evidenced a significant increase of mineralized tissue formed ectopically with time of implantation and allowed us to select the best ratio of HA to polysaccharides of 30% (w/w). Then, two Sr-substitution of 8% and 50% were incorporated in the HA powders (8Sr-HA and 50Sr-HA). Both Sr-HA were chemically characterized and dispersed in matrices. In vitro studies performed with human mesenchymal stem cells (MSCs) demonstrated the absence of cytotoxicity of the Sr-doped matrices whatever the amount of incorporated Sr. They also supported osteoblastic differentiation and activated the expression of one late osteoblastic marker involved in the mineralization process i.e. osteopontin. In vivo, subcutaneous implantation of these Sr-doped matrices induced osteoid tissue and blood vessels formation.
Conflict of interest statement
Figures



Similar articles
-
Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model.Colloids Surf B Biointerfaces. 2018 Mar 1;163:346-354. doi: 10.1016/j.colsurfb.2017.12.048. Epub 2017 Dec 28. Colloids Surf B Biointerfaces. 2018. PMID: 29331906
-
Intermittent administration of human parathyroid hormone (1-34) increases fixation of strontium-doped hydroxyapatite coating titanium implants via electrochemical deposition in ovariectomized rat femur.J Biomater Appl. 2016 Feb;30(7):952-60. doi: 10.1177/0885328215610898. Epub 2015 Oct 18. J Biomater Appl. 2016. PMID: 26482573
-
Osteogenesis of rat mesenchymal stem cells and osteoblastic cells on strontium-doped nanohydroxyapatite-coated titanium surfaces.Int J Oral Maxillofac Implants. 2015 Mar-Apr;30(2):461-71. doi: 10.11607/jomi.3798. Int J Oral Maxillofac Implants. 2015. PMID: 25830407
-
Strontium in the bone-implant interface.Dan Med Bull. 2011 May;58(5):B4286. Dan Med Bull. 2011. PMID: 21535993 Review.
-
Metal Ion-Doped Hydroxyapatite-Based Materials for Bone Defect Restoration.Bioengineering (Basel). 2023 Nov 28;10(12):1367. doi: 10.3390/bioengineering10121367. Bioengineering (Basel). 2023. PMID: 38135958 Free PMC article. Review.
Cited by
-
Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications.Materials (Basel). 2019 Nov 10;12(22):3704. doi: 10.3390/ma12223704. Materials (Basel). 2019. PMID: 31717621 Free PMC article. Review.
-
Therapeutic approaches to activate the canonical Wnt pathway for bone regeneration.J Tissue Eng Regen Med. 2022 Nov;16(11):961-976. doi: 10.1002/term.3349. Epub 2022 Sep 16. J Tissue Eng Regen Med. 2022. PMID: 36112528 Free PMC article. Review.
-
Does the incorporation of strontium into calcium phosphate improve bone repair? A meta-analysis.BMC Oral Health. 2022 Mar 8;22(1):62. doi: 10.1186/s12903-022-02092-7. BMC Oral Health. 2022. PMID: 35260122 Free PMC article.
-
Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: A review.Jpn Dent Sci Rev. 2022 Nov;58:233-248. doi: 10.1016/j.jdsr.2022.07.002. Epub 2022 Aug 27. Jpn Dent Sci Rev. 2022. PMID: 36065207 Free PMC article. Review.
-
Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes.Int J Mol Sci. 2022 Nov 29;23(23):14987. doi: 10.3390/ijms232314987. Int J Mol Sci. 2022. PMID: 36499315 Free PMC article. Review.
References
-
- Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN, et al. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:98–103. . - PubMed
-
- Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: What they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574–9. doi: 10.1302/0301-620X.89B5.19039 . - DOI - PubMed
-
- Hutmacher DW, Schantz JT, Lam CX, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1(4):245–60. doi: 10.1002/term.24 . - DOI - PubMed
-
- Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv Drug Deliv Rev. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

